Marmoset glutathione transferases with ketosteroid isomerase activity
- PMID: 34286113
- PMCID: PMC8280513
- DOI: 10.1016/j.bbrep.2021.101078
Marmoset glutathione transferases with ketosteroid isomerase activity
Abstract
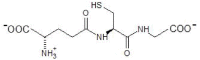
The common marmoset Callithrix jacchus encodes two glutathione transferase (GST) enzymes with ketosteroid double-bond isomerase activity. The most active enzyme is CjaGST A3-3 showing a specific activity with 5-androsten-3,17-dione (Δ5-AD) of 62.1 ± 1.8 μmol min-1 mg-1, and a kcat value of 261 ± 49 s-1. The second ketosteroid isomerase CjaGST A1-1 has a 30-fold lower specific activity with Δ5-AD and a 37-fold lower kcat value. Thus, the marmoset CjaGST A3-3 would be the main contributor to the biosynthesis of the steroid hormones testosterone and progesterone, like the human ortholog HsaGST A3-3. Two residues differ in the H-site of the 91.4% sequence identical CjaGST A1-1 and CjaGST A3-3, and modeling of the structures suggests that the bulky phenyl ring of Phe111 in CjaGST A1-1 causes steric hindrance in the binding of the steroid substrate. Tributyltin acetate (IC50=0.16 ± 0.004 μM) and ethacrynic acid (IC50=3.3 ± 0.2 μM) were found to be potent inhibitors of CjaGST A3-3, as previously demonstrated with the human and equine orthologs.
Keywords: 1-chloro-2,4-dinitrobenzene, (CDNB); 4-androsten-3,17-dione, (Δ4-AD); 5-Androsten-3,17-dione; 5-Pregnen-3,20-dione; 5-androsten-3,17-dione, (Δ5-AD); 5-pregnen-3,20-dione, (Δ5-PD); Alpha glutathione transferase; CjaGST A1-1; CjaGST A3-3; Glutathione transferase, (GST); Glutathione, (GSH); SDS-PAGE, (sodium dodecyl sulfate-polyacrylamide gel electrophoresis); Steroid hormone synthesis; allyl isothiocyanate, (AITC); phenethyl isothiocyanate, (PEITC).
© 2021 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

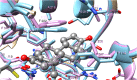


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

