Functional hydrogels for diabetic wound management
- PMID: 34286170
- PMCID: PMC8272650
- DOI: 10.1063/5.0046682
Functional hydrogels for diabetic wound management
Abstract
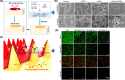
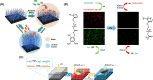
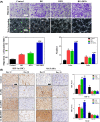
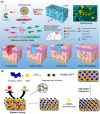
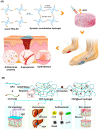
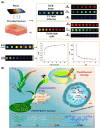
Diabetic wounds often have a slow healing process and become easily infected owing to hyperglycemia in wound beds. Once planktonic bacterial cells develop into biofilms, the diabetic wound becomes more resistant to treatment. Although it remains challenging to accelerate healing in a diabetic wound due to complex pathology, including bacterial infection, high reactive oxygen species, chronic inflammation, and impaired angiogenesis, the development of multifunctional hydrogels is a promising strategy. Multiple functions, including antibacterial, pro-angiogenesis, and overall pro-healing, are high priorities. Here, design strategies, mechanisms of action, performance, and application of functional hydrogels are systematically discussed. The unique properties of hydrogels, including bactericidal and wound healing promotive effects, are reviewed. Considering the clinical need, stimuli-responsive and multifunctional hydrogels that can accelerate diabetic wound healing are likely to form an important part of future diabetic wound management.
© 2021 Author(s).
Figures
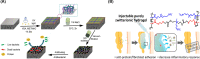


References
-
- Gao D., Mi Y., and Gao Z., “ Green approaches for the fabrication of electrospun poly (vinyl alcohol) nanofibers loaded epidermal growth factor derivative,” Mater. Lett. 276, 128237 (2020). 10.1016/j.matlet.2020.128237 - DOI
Publication types
LinkOut - more resources
Full Text Sources

