Impact of 18F-FDG-PET/CT on Clinical Management in Patients with Cholangiocellular Carcinoma
- PMID: 34286178
- PMCID: PMC8256695
- DOI: 10.1259/bjro.20210008
Impact of 18F-FDG-PET/CT on Clinical Management in Patients with Cholangiocellular Carcinoma
Abstract
Objective: To determine the impact of 18F-FDG-PET/CT on clinical management of patients with cholangiocellular carcinoma (CCA).
Methods: Patients with CCA undergoing clinically indicated 18F-FDG-PET/CT between 04/2013 and 08/2018 were prospectively included in a local PET/CT registry study. Intended clinical management ("non-treatment" such as watchful-waiting or additional diagnostic tests, and "palliative" or "curative treatment") was recorded before and after PET/CT. Changes in intended management after PET/CT were analyzed.
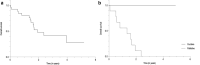
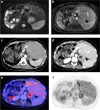
Results: 27 patients (mean age: 60 years, IQR: 51.5-67.5 years, 56% males) with 43 PET/CT examinations were included. Intended management changed in 35/43 cases (81.4%) following PET/CT. Major changes (i.e., between "non-treatment" and "treatment" strategies or between a "curative" and "palliative" treatment goal) occurred in 27/43 (62.8%) cases. Before PET/CT, additional imaging and/or biopsy were intended in 21/43 (48.8%) and 9/43 (20.9%) cases, respectively. After PET/CT, further imaging was carried out in one case and imaging-targeted biopsy in eight cases. Although the absolute number of biopsies after PET/CT did not decrease, in only one of these eight cases biopsy had already been planned before PET/CT, whereas in the other eight cases, the originally planned biopsies were dispensable after PET/CT.
Conclusions: 18F-FDG-PET/CT significantly impacts clinical management of patients with CCA. It guides decisions on treatment strategy (especially curative vs palliative treatment goal) and on additional tests, particularly by helping referring clinicians to avoid unnecessary imaging and by guiding targeted biopsy.
Advances in knowledge: Systematic implementation of 18F-FDG-PET/CT may enable a more appropriate and tailored treatment of patients with CCA, especially in cases of suspected recurrence.
© 2021 The Authors. Published by the British Institute of Radiology.
Figures



References
-
- Bragazzi MC, et al. Cholangiocarcinoma: epidemiology and risk factors. Transl. Gastrointest. Cancer 2011; 1: 21–32.
LinkOut - more resources
Full Text Sources

