Topographically guided hierarchical mineralization
- PMID: 34286238
- PMCID: PMC8273417
- DOI: 10.1016/j.mtbio.2021.100119
Topographically guided hierarchical mineralization
Abstract
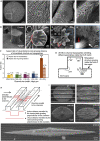
Material platforms based on interaction between organic and inorganic phases offer enormous potential to develop materials that can recreate the structural and functional properties of biological systems. However, the capability of organic-mediated mineralizing strategies to guide mineralization with spatial control remains a major limitation. Here, we report on the integration of a protein-based mineralizing matrix with surface topographies to grow spatially guided mineralized structures. We reveal how well-defined geometrical spaces defined within the organic matrix by the surface topographies can trigger subtle changes in single nanocrystal co-alignment, which are then translated to drastic changes in mineralization at the microscale and macroscale. Furthermore, through systematic modifications of the surface topographies, we demonstrate the possibility of selectively guiding the growth of hierarchically mineralized structures. We foresee that the capacity to direct the anisotropic growth of such structures would have important implications in the design of biomineralizing synthetic materials to repair or regenerate hard tissues.
Keywords: Bone; Crystallization; Dental enamel; Elastin-like recombinamer; Fluorapatite; Hierarchical mineralization; Protein-based biomineralization; Surface topographies.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Nudelman F., Sommerdijk N.A. Biomineralization as an inspiration for materials chemistry. Angew. Chem. Int. Ed. 2012;51(27):6582–6596. - PubMed
-
- Davis S.A., Dujardin E., Mann S. Biomolecular inorganic materials chemistry. Curr. Opin. Solid State Mater. Sci. 2003;7(4–5):273–281.
-
- Xu H., Smith D., Jahanmir S., Romberg E., Kelly J., Thompson V., Rekow E. Indentation damage and mechanical properties of human enamel and dentin. J. Dent. Res. 1998;77(3):472–480. - PubMed
-
- Boyde A. 1989. Enamel, Teeth; pp. 309–473. Springer.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

