Stem cell therapy for dilated cardiomyopathy
- PMID: 34286511
- PMCID: PMC8406792
- DOI: 10.1002/14651858.CD013433.pub2
Stem cell therapy for dilated cardiomyopathy
Abstract
Background: Stem cell therapy (SCT) has been proposed as an alternative treatment for dilated cardiomyopathy (DCM), nonetheless its effectiveness remains debatable.
Objectives: To assess the effectiveness and safety of SCT in adults with non-ischaemic DCM.
Search methods: We searched CENTRAL in the Cochrane Library, MEDLINE, and Embase for relevant trials in November 2020. We also searched two clinical trials registers in May 2020.
Selection criteria: Eligible studies were randomized controlled trials (RCT) comparing stem/progenitor cells with no cells in adults with non-ischaemic DCM. We included co-interventions such as the administration of stem cell mobilizing agents. Studies were classified and analysed into three categories according to the comparison intervention, which consisted of no intervention/placebo, cell mobilization with cytokines, or a different mode of SCT. The first two comparisons (no cells in the control group) served to assess the efficacy of SCT while the third (different mode of SCT) served to complement the review with information about safety and other information of potential utility for a better understanding of the effects of SCT.
Data collection and analysis: Two review authors independently screened all references for eligibility, assessed trial quality, and extracted data. We undertook a quantitative evaluation of data using random-effects meta-analyses. We evaluated heterogeneity using the I² statistic. We could not explore potential effect modifiers through subgroup analyses as they were deemed uninformative due to the scarce number of trials available. We assessed the certainty of the evidence using the GRADE approach. We created summary of findings tables using GRADEpro GDT. We focused our summary of findings on all-cause mortality, safety, health-related quality of life (HRQoL), performance status, and major adverse cardiovascular events.
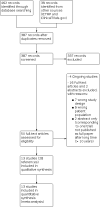
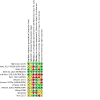
Main results: We included 13 RCTs involving 762 participants (452 cell therapy and 310 controls). Only one study was at low risk of bias in all domains. There were many shortcomings in the publications that did not allow a precise assessment of the risk of bias in many domains. Due to the nature of the intervention, the main source of potential bias was lack of blinding of participants (performance bias). Frequently, the format of the continuous data available was not ideal for use in the meta-analysis and forced us to seek strategies for transforming data in a usable format. We are uncertain whether SCT reduces all-cause mortality in people with DCM compared to no intervention/placebo (mean follow-up 12 months) (risk ratio (RR) 0.84, 95% confidence interval (CI) 0.54 to 1.31; I² = 0%; studies = 7, participants = 361; very low-certainty evidence). We are uncertain whether SCT increases the risk of procedural complications associated with cells injection in people with DCM (data could not be pooled; studies = 7; participants = 361; very low-certainty evidence). We are uncertain whether SCT improves HRQoL (standardized mean difference (SMD) 0.62, 95% CI 0.01 to 1.23; I² = 72%; studies = 5, participants = 272; very low-certainty evidence) and functional capacity (6-minute walk test) (mean difference (MD) 70.12 m, 95% CI -5.28 to 145.51; I² = 87%; studies = 5, participants = 230; very low-certainty evidence). SCT may result in a slight functional class (New York Heart Association) improvement (data could not be pooled; studies = 6, participants = 398; low-certainty evidence). None of the included studies reported major adverse cardiovascular events as defined in our protocol. SCT may not increase the risk of ventricular arrhythmia (data could not be pooled; studies = 8, participants = 504; low-certainty evidence). When comparing SCT to cell mobilization with granulocyte-colony stimulating factor (G-CSF), we are uncertain whether SCT reduces all-cause mortality (RR 0.46, 95% CI 0.16 to 1.31; I² = 39%; studies = 3, participants = 195; very low-certainty evidence). We are uncertain whether SCT increases the risk of procedural complications associated with cells injection (studies = 1, participants = 60; very low-certainty evidence). SCT may not improve HRQoL (MD 4.61 points, 95% CI -5.62 to 14.83; studies = 1, participants = 22; low-certainty evidence). SCT may improve functional capacity (6-minute walk test) (MD 140.14 m, 95% CI 119.51 to 160.77; I² = 0%; studies = 2, participants = 155; low-certainty evidence). None of the included studies reported MACE as defined in our protocol or ventricular arrhythmia. The most commonly reported outcomes across studies were based on physiological measures of cardiac function where there were some beneficial effects suggesting potential benefits of SCT in people with non-ischaemic DCM. However, it is unclear if this intermediate effects translates into clinical benefits for these patients. With regard to specific aspects related to the modality of cell therapy and its delivery, uncertainties remain as subgroup analyses could not be performed as planned, making it necessary to wait for the publication of several studies that are currently in progress before any firm conclusion can be reached.
Authors' conclusions: We are uncertain whether SCT in people with DCM reduces the risk of all-cause mortality and procedural complications, improves HRQoL, and performance status (exercise capacity). SCT may improve functional class (NYHA), compared to usual care (no cells). Similarly, when compared to G-CSF, we are also uncertain whether SCT in people with DCM reduces the risk of all-cause mortality although some studies within this comparison observed a favourable effect that should be interpreted with caution. SCT may not improve HRQoL but may improve to some extent performance status (exercise capacity). Very low-quality evidence reflects uncertainty regarding procedural complications. These suggested beneficial effects of SCT, although uncertain due to the very low certainty of the evidence, are accompanied by favourable effects on some physiological measures of cardiac function. Presently, the most effective mode of administration of SCT and the population that could benefit the most is unclear. Therefore, it seems reasonable that use of SCT in people with DCM is limited to clinical research settings. Results of ongoing studies are likely to modify these conclusions.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
The performance of this review was free of any real or perceived bias introduced by receipt of any benefit in cash or kind, or any subsidy derived from any source that may have or be perceived to have an interest in the outcomes of the review.
RD: none.
GU: has participated in several educational activities (workshops) and has provided consultancy to several laboratories, but has not been paid for it (money was paid to the institution where he works: Clinical Epidemiology Service, at the Hospital de la Santa Creu i Sant Pau).
JC: has consulted widely with other healthcare companies (Amgen, Novartis, Stealth Biopharmaceuticals, Bayer, Philips, Servier, Pharma Nord, Pharmacosmos, Vifor, HeartFelt Technologies, PharmaIn and Viscardia) but not on the topic of stem cells. He also received payments from the European Society of Cardiology for the development of educational presentations.
DP: none.
FV: none.
RAD: none.
SB: none.
GR: none.
EM: none.
Figures














Update of
- doi: 10.1002/14651858.CD013433
Similar articles
-
Tailored or adapted interventions for adults with chronic obstructive pulmonary disease and at least one other long-term condition: a mixed methods review.Cochrane Database Syst Rev. 2021 Jul 26;7(7):CD013384. doi: 10.1002/14651858.CD013384.pub2. Cochrane Database Syst Rev. 2021. PMID: 34309831 Free PMC article.
-
Interventions for preventing and treating cardiac complications in Duchenne and Becker muscular dystrophy and X-linked dilated cardiomyopathy.Cochrane Database Syst Rev. 2018 Oct 16;10(10):CD009068. doi: 10.1002/14651858.CD009068.pub3. Cochrane Database Syst Rev. 2018. PMID: 30326162 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 May 20;5(5):CD013600. doi: 10.1002/14651858.CD013600.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Feb 1;2:CD013600. doi: 10.1002/14651858.CD013600.pub5. PMID: 34013969 Free PMC article. Updated.
-
Psychosocial interventions for preventing and treating depression in dialysis patients.Cochrane Database Syst Rev. 2019 Dec 2;12(12):CD004542. doi: 10.1002/14651858.CD004542.pub3. Cochrane Database Syst Rev. 2019. PMID: 31789430 Free PMC article.
-
Digital interventions for the management of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD013246. doi: 10.1002/14651858.CD013246.pub2. Cochrane Database Syst Rev. 2021. PMID: 33871065 Free PMC article.
Cited by
-
Six-minute walk test as clinical end point in cardiomyopathy clinical trials, including ATTR-CM: a systematic literature review.J Comp Eff Res. 2024 Jul;13(7):e230158. doi: 10.57264/cer-2023-0158. Epub 2024 Jun 13. J Comp Eff Res. 2024. PMID: 38869839 Free PMC article.
-
A correspondence on efficacy and safety of stem cell therapy in patients with dilated cardiomyopathy.Int J Surg. 2024 Sep 1;110(9):5989-5990. doi: 10.1097/JS9.0000000000001743. Int J Surg. 2024. PMID: 39275781 Free PMC article. No abstract available.
-
Exploring novel biomarkers in dilated cardiomyopathy‑induced heart failure by integrated analysis and in vitro experiments.Exp Ther Med. 2023 May 16;26(1):325. doi: 10.3892/etm.2023.12024. eCollection 2023 Jul. Exp Ther Med. 2023. PMID: 37346398 Free PMC article.
-
Mass Customized Outlook for Regenerative Heart Failure Care.Int J Mol Sci. 2021 Oct 22;22(21):11394. doi: 10.3390/ijms222111394. Int J Mol Sci. 2021. PMID: 34768825 Free PMC article. Review.
-
What are the best clinical management strategies for cardiomyopathy? an umbrella review of systematic reviews and meta-analyses.Front Pharmacol. 2025 Jul 25;16:1544121. doi: 10.3389/fphar.2025.1544121. eCollection 2025. Front Pharmacol. 2025. PMID: 40786045 Free PMC article.
References
References to studies included in this review
Hamshere 2015 {published data only}
-
- Arnous S, Mozid A, Mathur A. The Bone Marrow Derived Adult Stem Cells for Dilated Cardiomyopathy (REGENERATE-DCM) trial: study design. Regenerative Medicine 2011;6(4):525-33. - PubMed
-
- EUCTR2009-013112-12-GB. Randomised controlled trial to compare the effects of G-CSF(Granocyte™) and autologous bone marrow progenitor cells on quality of life and left ventricular function In patients with idiopathic dilated cardiomyopathy – REGENERATE-DCM. ddrare.nibiohn.go.jp/cgi-bin/disease_who_e.cgi?id=57 (accessed prior to 1 July 2021).
-
- Hamshere S, Arnous S, Choudhury T, Choudry F, Mozid A, Yeo C, et al. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: the REGENERATE-DCM clinical trial. European Heart Journal 2015;36(44):3061-9. [DOI: ] - PMC - PubMed
-
- NCT01302171. Bone Marrow Derived Adult Stem Cells for Dilated Cardiomyopathy (REGEN-DCM). clinicaltrials.gov/ct2/show/NCT01302171 (first received 24 February 2011).
Hare 2017 (POSEIDON‐DCM) {published data only}
-
- Mushtaq M, DiFede DL, Golpanian S, Khan A, Gomes SA, Mendizabal A, et al. Rationale and design of the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy (the POSEIDON-DCM study): a phase I/II, randomized pilot study of the comparative safety and efficacy of transendocardial injection of autologous mesenchymal stem cell vs. allogeneic mesenchymal stem cells in patients with non-ischemic dilated cardiomyopathy. Journal of Cardiovascular Translational Research 2014;7(9):769-80. - PMC - PubMed
-
- Ramireddy A, Brodt CR, Mendizabal AM, DiFede DL, Healy C, Goyal V, et al. Effects of transendocardial stem cell injection on ventricular proarrhythmia in patients with ischemic cardiomyopathy: results from the POSEIDON and TAC-HFT Trials. Stem Cells Translational Medicine 2017;6:1366-72. [DOI: 10.1002/sctm.16-0328] - DOI - PMC - PubMed
Henry 2014 {published data only}
-
- Bruckner BA, Ghodsizad A, Hamman BL, Bull DA, Lattouf OM, Smedira NG, et al. IMPACT-DCM: a randomized, controlled, multi-center phase II trial utilizing expanded autologous bone marrow as sole therapy for dilated cardiomyopathy study update. Journal of Heart and Lung Transplantation 2011;30:79.
-
- Bunge RR, Patel AN, Hamman BL, Lattouf OM, Smedira NG, Bartel RL, et al. Safety and efficacy of ixmyelocel-t, an expanded patient-specific mixed cell product, in dilated cardiomyopathy (IMPACT-DCM). Journal of Heart and Lung Transplantation 2012;31:72-3.
-
- Patel AN, Hamman BL, Bruckner B, Lattouf OM, Smedira NG, East C, et al. Safety and efficacy of ixmyelocel-T, an expanded patient-specific mixed cell product, in dilated cardiomyopathy (IMPACT-DCM). Journal of Cardiac Failure 2011;17:58.
Martino 2015 (MiHEART) {published data only}
-
- Martino H, Brofman P, Greco O, Bueno R, Bodanese L, Clausell N, et al. Multicentre, randomized, double-blind trial of intracoronary autologous mononuclear bone marrow cell injection in non-ischaemic dilated cardiomyopathy (the dilated cardiomyopathy arm of the MiHeart study). European Heart Journal 2015;36(42):2898-904. - PubMed
Sant'Anna 2014 (INTRACELL) {published data only}
-
- Sant'Anna RT, Fracasso J, Valle FH, Castro I, Nardi NB, Sant'Anna JR, et al. Direct intramyocardial transthoracic transplantation of bone marrow mononuclear cells for non-ischemic dilated cardiomyopathy: INTRACELL, a prospective randomized controlled trial. Revista Brasileira de Cirurgia Cardiovascular 2014;29(3):437-47. - PMC - PubMed
Seth 2010 (ABCD) {published data only}
-
- Seth S, Bhargava B, Narang R, Mohanty S, Ray R, Gulati G, et al. A randomised trial of Autologous Bone marrow Cells in Dilated cardiomyopathy (ABCD). European Heart Journal 2009;30:501.
-
- Seth S, Bhargava B, Narang R, Ray R, Mohanty S, Gulati G, et al. The ABCD (Autologous Bone Marrow Cells in Dilated Cardiomyopathy) trial: a long-term follow-up study. Journal of the American College of Cardiology 2010;55(15):1643-4. - PubMed
-
- Seth S, Narang R, Bhargava B, Ray R, Mohanty S, Gulati G, et al. Percutaneous intracoronary cellular cardiomyoplasty for nonischemic cardiomyopathy: clinical and histopathological results: the first-in-man ABCD (Autologous Bone marrow Cells in Dilated cardiomyopathy) trial. Journal of the American College of Cardiology 2006;48(11):2350-1. - PubMed
Vrtovec 2011 {published data only}
-
- Vrtovec B, Poglajen G, Sever M, Lezaic L, Domanovic D, Cernelc P, et al. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. Journal of Cardiac Failure 2011;17(4):272-81. [DOI: ] - PubMed
Vrtovec 2013a (NOGA‐DCM) {published data only}
-
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, et al. Effects of intracoronary CD34+stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circulation Research 2013;112(1):165-73. - PubMed
-
- Vrtovec B, Sever M, Domanovic D, Lezaic L, Poglajen G, Cernelc P, et al. Long-term effects of stem cell transplantation in heart failure. Zdravniski Vestnik 2012;81(Suppl II):373-83.
Vrtovec 2013b {published data only}
-
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, et al. Comparison of clinical effects of transendocardial vs. intracoronary stem cell transplantation in dilated cardiomyopathy. Circulation 2012;126:21. - PubMed
-
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation 2013;128(11):S42-9. - PubMed
Vrtovec 2018 (REMEDIUM) {published data only}
-
- Vrtovec B, Poglajen G, Sever M, Zemljic G, Frljak S, Cerar A, et al. Effects of repetitive transendocardial CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation Research 2018;123(3):389-96. - PubMed
Wang 2006 {published data only}
-
- Wang JA, Xie XJ, He H, Sun Y, Jiang J, Luo RH, et al. A prospective, randomized, controlled trial of autologous mesenchymal stem cells transplantation for dilated cardiomyopathy. Chung Hua Hsin Hsueh Kuan Ping Tsa Chih 2006;34(2):107-10. - PubMed
Wu 2010 {published data only}
-
- Wu ZH, Yuan MY, Li HM, Qiu JJ, Lao HZ, Wu XY, et al. Autologous peripheral blood stem cells transplantation for the treatment of dilated cardiomyopathy: a 24-month follow-up in 38 cases. Journal of Clinical Rehabilitative Tissue Engineering Research 2010;14(1):121-25. [DOI: ]
Xiao 2017 {published data only}
-
- Xiao W, Guo S, Gao C, Dai G, Gao Y, Li M, et al. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. International Heart Journal 2017;58(2):238-44. [DOI: ] - PubMed
References to studies excluded from this review
Bartolucci (INNOVA) 2015 {published data only}
-
- Bartolucci J, Verdugo FJ, Carrion F, Abarzúa E, Goset C, Lamich R, et al. Long-term results of intracoronary transplantation of autologous bone marrow cells in dilated cardiomyopathy [Resultados a largo plazo deltrasplante intracoronario de células mononucleares de médula ósea autólogas enpacientes con cardiopatía dilatada de diversa etiología]. Revista Médica de Chile 2015;143(4):415-23. - PubMed
Bartolucci (RIMECARD) 2017 {published data only}
-
- Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [Randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circulation Research 2017;121(10):1192-204. - PMC - PubMed
Bocchi 2010 {published data only}
-
- Bocchi EA, Bacal F, Guimaraes G, Mendroni A, Mocelin A, Filho AE, et al. Granulocyte-colony stimulating factor or granulocyte-colony stimulating factor associated to stem cell intracoronary infusion effects in non ischemic refractory heart failure. International Journal of Cardiology 2010;138(1):94-7. [DOI: ] - PubMed
Butler 2017 {published data only}
-
- Butler J, Epstein SE, Greene SJ, Quyyumi AA, Sikora S, Kim RJ, et al. Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy: safety and efficacy results of a phase II – a randomized trial. Circulation Research 2017;120(2):332-40. - PubMed
Chen 2008 {published data only}
-
- Chen Y, Gao EM, Gao CY, Xu Y, Huang KJ, Niu ZM, et al. Effects of intracoronary autologous bone marrow mononuclear cells transplantation in patients with dilated cardiomyopathy. Zhonghua Xin Xue GuanBing Za Zhi 2008;36(12):1087-91. - PubMed
Fischer‐Rasokat 2009 {published data only}
-
- Fischer-Rasokat U, Assmus B, Seeger FH, Honold J, Leistner D, Fichtlscherer S, et al. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circulation. Heart Failure 2009;2(5):417-23. - PubMed
Huang 2006 {published data only}
-
- Huang RC, Yao K, Li YL, Zhang YQ, Xu SK, Shi HY, et al. Transplantation of autologous bone marrow mononuclear cells on patients with idiopathic dilated cardiomyopathy: early results on effect and security. Zhonghua Xin Xue Guan Bing Za Zhi 2006;34(2):111-3. - PubMed
Kakuchaya 2011 {published data only}
-
- Kakuchaya T, Golukhova E, Eremeeva M, Chigogidze N, Aslanidi I, Nikitina T, et al. Bone-marrow progenitor stem cells for the treatment of patients with congestive heart failure of different etiology in a placebo controlled clinical trial. Interactive Cardiovascular and Thoracic Surgery 2011;12:568.
-
- Kakuchaya T, Golukhova E, Eremeeva M, Chigogidze N, Aslanidi I, Shurupova I, et al. Accurate design of randomized placebo-controlled clinical trials for assessment of stem cell effects on cardiac regeneration. European Heart Journal 2011;32:290.
Miyagawa 2017 {published data only}
NCT02256501 {unpublished data only}
-
- NCT02256501. Intracoronary transplantation of bone marrow derived mononuclear cells in pediatric cardiomyopathy. clinicaltrials.gov/ct2/show/NCT02256501 (first received 3 October 2014).
Perin (REVASCOR) 2015 {published data only}
-
- Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, et al. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circulation Research 2015;117(6):576-84. - PubMed
Premer 2015 {published data only}
Tompkins 2018 {published data only}
Xiao 2012a {published data only}
-
- Xiao WT, Gao LJ, Gao CY, Gao YJ, Dai GY, Li MW, et al. Comparative study on the efficacy of intracoronary infusion with various types of autologous bone marrow stem cells for patients with dilated cardiomyopathy. Zhonghua Xin XueGuan Bing Za Zhi 2012;40(7):575-8. - PubMed
Xiao 2012b {published data only}
-
- Xiao WT, Gao CY, Dai GY, Li MW, Wang XP, Liu HZ, et al. Autologous bone marrow mesenchymal stem cells for myocardial renewal and repair. Chinese Journal of Tissue Engineering Research 2012;16(27):5081-6.
Yau 2019 {published data only}
-
- Yau TM, Pagani FD, Mancini DM, Chang HL, Lala A, Woo YJ, et al. Intramyocardial injection of mesenchymal precursor cells and successful temporary weaning from left ventricular assist device support in patients with advanced heart failure: a randomized clinical trial. JAMA 2019;321(12):1176-86. - PMC - PubMed
Zemljic 2017 {published data only}
-
- Zemljic G, Poglajen G, Sever M, Cukjati M, Frljak S, Androcec V, et al. Electroanatomic properties of the myocardium predict response to CD34+ cell therapy in patients with ischemic and nonischemic heart failure. Journal of Cardiac Failure 2017;23(2):153-60. - PubMed
References to ongoing studies
NCT01957826 {published data only}
-
- NCT01957826. Mesenchymal stem cells for idiopathic dilated cardiomyopathy. clinicaltrials.gov/ct2/show/NCT01957826 (first received 8 October 2013).
NCT02033278 {published data only}
-
- NCT02033278. Infusion intracoronary of mononuclear autologous adult no expanded stem cells of bone marrow on functional recovery in patients with idiopathic dilated cardiomyopathy and heart failure. clinicaltrials.gov/ct2/show/NCT02033278 (first received 10 January 2014).
NCT02293603 {published data only}
-
- NCT02293603. Dilated cardiomYopathy iNtervention With Allogeneic MyocardIally-regenerative Cells (DYNAMIC). clinicaltrials.gov/ct2/show/NCT02293603 (first received 18 November 2014).
NCT03797092 {published data only}
-
- NCT03797092. Stem cell therapy in non-ischemic non-treatable dilated cardiomyopathies II: a pilot study. clinicaltrials.gov/ct2/show/NCT03797092 (first received 8 January 2019).
Additional references
Assmus 2002
-
- Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation 2002;106(24):3009-17. - PubMed
Assmus 2016
Bartunek 2013
-
- Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. Journal of the American College of Cardiology 2013;61(23):2329-38. - PubMed
Bartunek 2016
Bartunek 2017
-
- Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. European Heart Journal 2017;38(9):648-60. - PMC - PubMed
Bartunek 2018
Behfar 2014
-
- Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ. Cell therapy for cardiac repair-lessons from clinical trials. Nature Reviews. Cardiology 2014;11:232-46. - PubMed
Behfar 2016
-
- Behfar A, Gersh B, Terzic A. Repetition rescues regenerative reserve. European Heart Journal 2016;37(21):1667-70. - PubMed
Blau 2001
-
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell 2001;105(7):829-41. - PubMed
Blau 2019
-
- Blau HM, Daley GQ. Stem cells in the treatment of disease. New England Journal of Medicine 2019;380(18):1748-60. - PubMed
Bozkurt 2016
-
- Bozkurt B, Colvin M, Cook J, Cooper JT, Deswal A, Fonarow GC, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation 2016;134:e579-646. - PubMed
Chien 2019
-
- Chien KR, Frisén J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL. Regenerating the field of cardiovascular cell therapy. Nature Biotechnology 2019;37(3):232-7. - PubMed
Choudry 2016
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioural Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988.
Covidence [Computer program]
-
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, accessed prior to 1 July 2021. Available at covidence.org.
Dec 1994
-
- Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. New England Journal of Medicine 1994;331:1564-75. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. - PubMed
Díaz 1987
Dunlay 2012
Egger 1997
Erbs 2005
-
- Erbs S, Linke A, Adams V, Lenk K, Thiele H, Diederich KW, et al. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion: first randomized and placebo-controlled study. Circulation Research 2005;97(8):756-62. - PubMed
Fisher 2015
Fisher 2016a
Fisher 2016b
-
- Fisher SA, Doree C, Taggart DP, Mathur A, Martin-Rendon E. Cell therapy for heart disease: trial sequential analyses of two Cochrane reviews. Clinical Pharmacology and Therapeutics 2016;100(1):88-101. - PubMed
Frljak 2018
Fuster 1981
-
- Fuster V, Gersh BJ, Tajik AJ, Brandenburg RO, Frye RL. The natural history of idiopathic dilated cardiomyopathy. American Journal of Cardiology 1981;47(3):525-31. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed prior to 11 September 2019. Available at gradepro.org.
Harada 2005
-
- Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nature Medicine 2005;11:305-11. - PubMed
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. - PubMed
Hare 2009
-
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology 2009;54(24):2277-86. - PMC - PubMed
Hare 2017
Hershberger 2013
-
- Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nature Reviews. Cardiology 2013;10(9):531-47. - PubMed
Higgins 2011
-
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Jefferies 2010
-
- Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet 2010;375(9716):752-62. - PubMed
Jiao 2014
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Liberati 2009
Lu 2016
Marquis‐Gravel 2014
-
- Marquis-Gravel G, Stevens LM, Manosur S, Avram R, Noiseux N. Stem cell therapy for the treatment of nonischemic cardiomyopathy: a systematic review of the literature and meta-analysis of randomized controlled trials. Canadian Journal of Cardiology 2014;30(11):1378-84. - PubMed
McKenna 2017
-
- McKenna WJ, Maron BJ, Thiene G. Classification, epidemiology, and global burden of cardiomyopathies. Circulation Research 2017;121:722-30. - PubMed
Menasché 2001
-
- Menasché P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet 2001;357(9252):279-80. - PubMed
Menasché 2018
-
- Menasché P. Cell therapy trials for heart regeneration-lessons learned and future directions. Nature Reviews. Cardiology 2018;15(11):659-71. - PubMed
Merlo 2014
-
- Merlo M, Pivetta A, Pinamonti B, Stolfo D, Zecchin M, Barbati G, et al. Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: changing mortality over the last 30 years. European Journal of Heart Failure 2014;16(3):317-24. - PubMed
Merlo 2015
-
- Merlo M, Stolfo D, Anzini M, Negri F, Pinamonti B, Barbati G, et al. Persistent recovery of normal left ventricular function and dimension in idiopathic dilated cardiomyopathy during long-term follow-up: does real healing exist? Journal of the American Heart Association 2015;4(1):e001504. - PMC - PubMed
Merlo 2016
-
- Merlo M, Cannatá A, Vitagliano A, Zambon E, Lardieri G, Sinagra G. Clinical management of dilated cardiomyopathy: current knowledge and future perspectives. Expert Review of Cardiovascular Therapy 2016;14(2):137-40. - PubMed
Patel 2015
Pinto 2016
-
- Pinto YM, Elliot PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. European Heart Journal 2016;37(23):1850-8. - PubMed
Poglagen 2018
Ponikowski 2016
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 2016;37:2129-200. - PubMed
Psaltis 2010
-
- Psaltis PJ, Zannettino AC, Gronthos S, Worthley SG. Intramyocardial navigation and mapping for stem cell delivery. Journal of Cardiovascular Translational Research 2010;3:135-46. - PubMed
R [Computer program]
-
- R Foundation for Statistical Computing R: a language and environment for statistical computing. Version 3.4.2. Vienna, Austria: R Foundation for Statistical Computing, 2017. Available at www.R-project.org.
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rong 2019
Schulz 2010
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Stamm 2003
-
- Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003;361:45-6. - PubMed
Stehlik 2011
-
- Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, et al. The registry of the International Society of Heart and Lung Transplantation: twenty-eight adult heart transplantation report. Journal of Heart and Lung Transplantation 2011;30(10):1078-94. - PubMed
Strauer 2002
-
- Strauer BE, Brehm M, Zeus T, Köstering M, Hernadez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106:1913-8. - PubMed
Terzic 2016
-
- Terzic A, Behfar A. CardioPulse: regenerative medicine in the practice of cardiology. European Heart Journal 2016;37(14):1089-90. - PubMed
Terzic 2017
Vrtovec 2013
-
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circulation Research 2013;112:165-73. - PubMed
Vrtovec 2018a
-
- Vrtovec B. Cell therapy for nonischemic cardiomyopathy: current status and future perspectives. Circulation Research 2018;5(122):28-30. - PubMed
Vrtovec 2018b
-
- Vrtovec B, Poglajen G, Sever M, Zemljic G, Frljak S, Cerar A, et al. Effects of repetitive transendocardial CD34(+) cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation Research 2018;123:389-96. - PubMed
Weintraub 2017
-
- Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet 2017;390(10092):400-41. - PubMed
Wen 2018
-
- Wen Y, Ding J, Zhang B, Gao Q. Bone marrow-derived mononuclear cell therapy for nonischaemic dilated cardiomyopathy. A meta-analysis. European Journal of Clinical Investigation 2018;48(4):e12894. - PubMed
WHO/ISFC 1980
Yamada 2020
Yancy 2013
-
- Yancy C, Jessup M, Bozkurt B, Butler J, Casey D, Drazner M, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128(16):e240-27. - PubMed

