Effects of Patient Characteristics on Diagnostic Performance of Self-Collected Samples for SARS-CoV-2 Testing
- PMID: 34286681
- PMCID: PMC8314823
- DOI: 10.3201/eid2708.210667
Effects of Patient Characteristics on Diagnostic Performance of Self-Collected Samples for SARS-CoV-2 Testing
Abstract
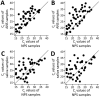
We evaluated the performance of self-collected anterior nasal swab (ANS) and saliva samples compared with healthcare worker-collected nasopharyngeal swab specimens used to test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We used the same PCR diagnostic panel to test all self-collected and healthcare worker-collected samples from participants at a public hospital in Atlanta, Georgia, USA. Among 1,076 participants, 51.9% were men, 57.1% were >50 years of age, 81.2% were Black (non-Hispanic), and 74.9% reported >1 chronic medical condition. In total, 8.0% tested positive for SARS-CoV-2. Compared with nasopharyngeal swab samples, ANS samples had a sensitivity of 59% and saliva samples a sensitivity of 68%. Among participants tested 3-7 days after symptom onset, ANS samples had a sensitivity of 80% and saliva samples a sensitivity of 85%. Sensitivity varied by specimen type and patient characteristics. These findings can help physicians interpret PCR results for SARS-CoV-2.
Keywords: 2019 novel coronavirus disease; COVID-19; Georgia; SARS-CoV-2; United States; anterior nasal; coronavirus disease; coronaviruses; respiratory infections; saliva; self-collected; sensitivity; severe acute respiratory syndrome coronavirus 2; viruses; zoonoses.
Figures

References
-
- World Health Organization. COVID-19 weekly epidemiological update—27 January 2021. 2021 Jan 27 [cited 2021 Feb 9]. https://www.who.int/publications/m/item/weekly-epidemiological-update---...
-
- Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens for COVID-19. 2021. [cited 2021 Jan 29]. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

