SwabExpress: An End-to-End Protocol for Extraction-Free COVID-19 Testing
- PMID: 34286830
- PMCID: PMC8406859
- DOI: 10.1093/clinchem/hvab132
SwabExpress: An End-to-End Protocol for Extraction-Free COVID-19 Testing
Abstract
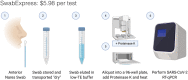
Background: The urgent need for massively scaled clinical testing for SARS-CoV-2, along with global shortages of critical reagents and supplies, has necessitated development of streamlined laboratory testing protocols. Conventional nucleic acid testing for SARS-CoV-2 involves collection of a clinical specimen with a nasopharyngeal swab in transport medium, nucleic acid extraction, and quantitative reverse-transcription PCR (RT-qPCR). As testing has scaled across the world, the global supply chain has buckled, rendering testing reagents and materials scarce. To address shortages, we developed SwabExpress, an end-to-end protocol developed to employ mass produced anterior nares swabs and bypass the requirement for transport media and nucleic acid extraction.
Methods: We evaluated anterior nares swabs, transported dry and eluted in low-TE buffer as a direct-to-RT-qPCR alternative to extraction-dependent viral transport media. We validated our protocol of using heat treatment for viral inactivation and added a proteinase K digestion step to reduce amplification interference. We tested this protocol across archived and prospectively collected swab specimens to fine-tune test performance.
Results: After optimization, SwabExpress has a low limit of detection at 2-4 molecules/µL, 100% sensitivity, and 99.4% specificity when compared side by side with a traditional RT-qPCR protocol employing extraction. On real-world specimens, SwabExpress outperforms an automated extraction system while simultaneously reducing cost and hands-on time.
Conclusion: SwabExpress is a simplified workflow that facilitates scaled testing for COVID-19 without sacrificing test performance. It may serve as a template for the simplification of PCR-based clinical laboratory tests, particularly in times of critical shortages during pandemics.
© American Association for Clinical Chemistry 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Update of
-
SwabExpress: An end-to-end protocol for extraction-free COVID-19 testing.bioRxiv [Preprint]. 2021 Apr 29:2020.04.22.056283. doi: 10.1101/2020.04.22.056283. bioRxiv. 2021. Update in: Clin Chem. 2021 Dec 30;68(1):143-152. doi: 10.1093/clinchem/hvab132. PMID: 32511368 Free PMC article. Updated. Preprint.
Comment in
-
A Push for Real Normal: Mass Screening for COVID-19.Clin Chem. 2021 Dec 30;68(1):4-6. doi: 10.1093/clinchem/hvab190. Clin Chem. 2021. PMID: 34617102 Free PMC article. No abstract available.
References
-
- Hellewell, J., Russell, T.W., The SAFER Investigators and Field Study Team. et al. Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections. BMC Med 19, 106 (2021). 10.1186/s12916-021-01982-x.et al. - DOI - PMC - PubMed
-
- Moore C, Corden S, Sinha J, Jones R.. Dry cotton or flocked respiratory swabs as a simple collection technique for the molecular detection of respiratory viruses using real-time NASBA. J Virol Methods 2008;153:84–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1RM1HG010461-01/Emergent Ventures
- NHGRI
- RM1 HG010461/HG/NHGRI NIH HHS/United States
- Gates Ventures
- T32 AI007044/AI/NIAID NIH HHS/United States
- The Seattle Flu Study and SCAN
- Security Act (CARES Act)
- HHMI/Howard Hughes Medical Institute/United States
- Ellume and Cepheid outside of the submitted work
- Sanofi Pasteur
- T32 GM007266/GM/NIGMS NIH HHS/United States
- Bill and Melinda Gates Foundation
- K24 AI150991/AI/NIAID NIH HHS/United States
- Brotman Baty Institute for Precision Medicine
- Testing for America supporting separate work on SwabSeq development
- University of Washington
- Ginkgo Bioworks supporting separate work on CLIA laboratory formation
- K24AI150991-01S1/NIH/NIAID
- HR001117S0019/HAARVI study comes from DARPA
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

