Clinical and genetic risk factors for radiation-associated ototoxicity: A report from the Childhood Cancer Survivor Study and the St. Jude Lifetime Cohort
- PMID: 34286861
- PMCID: PMC8516694
- DOI: 10.1002/cncr.33775
Clinical and genetic risk factors for radiation-associated ototoxicity: A report from the Childhood Cancer Survivor Study and the St. Jude Lifetime Cohort
Abstract
Background: Cranial radiation therapy (CRT) is associated with ototoxicity, which manifests as hearing loss and tinnitus. The authors sought to identify clinical determinants and genetic risk factors for ototoxicity among adult survivors of pediatric cancer treated with CRT.
Methods: Logistic regression evaluated associations of tinnitus (n = 1991) and hearing loss (n = 2198) with nongenetic risk factors and comorbidities among CRT-treated survivors in the Childhood Cancer Survivor Study. Genome-wide association studies (GWASs) of CRT-related tinnitus and hearing loss were also performed.
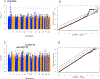
Results: Males were more likely to report CRT-related tinnitus (9.4% vs 5.4%; P = 5.1 × 10-4 ) and hearing loss (14.0% vs 10.7%; P = .02) than females. Survivors with tinnitus or hearing loss were more likely to experience persistent dizziness or vertigo (tinnitus: P < 2 × 10-16 ; hearing loss: P = 6.4 × 10-9 ), take antidepressants (tinnitus: P = .02; hearing loss: P = .01), and report poorer overall health (tinnitus: P = 1.5 × 10-6 ; hearing loss: P = 1.7 × 10-6 ) in comparison with controls. GWAS of CRT-related tinnitus revealed a genome-wide significant signal in chromosome 1 led by rs203248 (P = 1.5 × 10-9 ), whereas GWAS of CRT-related hearing loss identified rs332013 (P = 5.8 × 10-7 ) in chromosome 8 and rs67522722 (P = 7.8 × 10-7 ) in chromosome 6 as nearly genome-wide significant. A replication analysis identified rs67522722, intronic to ATXN1, as being significantly associated with CRT-related hearing loss (P = .03) and de novo hearing loss (P = 3.6 × 10-4 ).
Conclusions: CRT-associated ototoxicity was associated with sex, several neuro-otological symptoms, increased antidepressant use, and poorer self-reported health. GWAS of CRT-related hearing loss identified rs67522722, which was supported in an independent cohort of survivors.
Lay summary: Hearing loss and subjective tinnitus (the perception of noise or ringing in the ear) are long-term side effects of cancer treatment and are common in children treated with radiation to the brain. These toxicities can affect childhood development and potentially contribute to serious learning and behavioral difficulties. This study's data indicate that males are at greater risk for hearing loss and tinnitus than females after radiation therapy to the brain. Those who develop these toxicities are more likely to use antidepressants and report poorer overall health. Health care providers can improve the management of survivors by informing patients and/or their parents of these risks.
Keywords: genome-wide; ototoxicity; pediatric oncology; radiation.
© 2021 American Cancer Society.
Conflict of interest statement
Figures
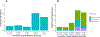
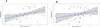
Similar articles
-
Prevalence and risk factors for ototoxicity after cisplatin-based chemotherapy.J Cancer Surviv. 2023 Feb;17(1):27-39. doi: 10.1007/s11764-022-01313-w. Epub 2023 Jan 13. J Cancer Surviv. 2023. PMID: 36637632
-
Pharmacogenomics of cisplatin-induced neurotoxicities: Hearing loss, tinnitus, and peripheral sensory neuropathy.Cancer Med. 2022 Jul;11(14):2801-2816. doi: 10.1002/cam4.4644. Epub 2022 Mar 23. Cancer Med. 2022. PMID: 35322580 Free PMC article.
-
Whole-Genome Sequencing of Childhood Cancer Survivors Treated with Cranial Radiation Therapy Identifies 5p15.33 Locus for Stroke: A Report from the St. Jude Lifetime Cohort Study.Clin Cancer Res. 2019 Nov 15;25(22):6700-6708. doi: 10.1158/1078-0432.CCR-19-1231. Epub 2019 Aug 28. Clin Cancer Res. 2019. PMID: 31462438 Free PMC article.
-
The long-term impacts of hearing loss, tinnitus and poor balance on the quality of life of people living with and beyond cancer after platinum-based chemotherapy: a literature review.J Cancer Surviv. 2023 Feb;17(1):40-58. doi: 10.1007/s11764-022-01314-9. Epub 2023 Jan 13. J Cancer Surviv. 2023. PMID: 36637633 Free PMC article. Review.
-
Cancer survivors treated with platinum-based chemotherapy affected by ototoxicity and the impact on quality of life: a narrative synthesis systematic review.Int J Audiol. 2019 Nov;58(11):685-695. doi: 10.1080/14992027.2019.1660918. Epub 2019 Sep 23. Int J Audiol. 2019. PMID: 31545660
Cited by
-
Machine learning-driven exploration of drug therapies for triple-negative breast cancer treatment.Front Mol Biosci. 2023 Aug 4;10:1215204. doi: 10.3389/fmolb.2023.1215204. eCollection 2023. Front Mol Biosci. 2023. PMID: 37602329 Free PMC article.
-
Remote Field Application of Digital Technology for Hearing Assessments in a Cohort of Pediatric Germ Cell Tumor Survivors.Cancer Epidemiol Biomarkers Prev. 2024 Sep 3;33(9):1177-1184. doi: 10.1158/1055-9965.EPI-24-0203. Cancer Epidemiol Biomarkers Prev. 2024. PMID: 38869488 Free PMC article.
-
Comparison of GWAS results between de novo tinnitus and cancer treatment-related tinnitus suggests distinctive roles for genetic risk factors.Sci Rep. 2024 Nov 14;14(1):27952. doi: 10.1038/s41598-024-78274-w. Sci Rep. 2024. PMID: 39543288 Free PMC article.
-
Ranking Breast Cancer Drugs and Biomarkers Identification Using Machine Learning and Pharmacogenomics.ACS Pharmacol Transl Sci. 2023 Feb 24;6(3):399-409. doi: 10.1021/acsptsci.2c00212. eCollection 2023 Mar 10. ACS Pharmacol Transl Sci. 2023. PMID: 36926455 Free PMC article.
-
Identification of Risk Loci for Radiotherapy-Induced Tinnitus and Hearing Loss Through Integrated Genomic Analysis.Int J Mol Sci. 2025 Apr 26;26(9):4132. doi: 10.3390/ijms26094132. Int J Mol Sci. 2025. PMID: 40362371 Free PMC article.
References
-
- Surveillance, Epidemiology, and End Results (SEER) Program released April 2019.www.seer.cancer.gov.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

