Improving vitamin D testing and supplementation in children with newly diagnosed cancer: A quality improvement initiative at Rady Children's Hospital San Diego
- PMID: 34286891
- PMCID: PMC8463415
- DOI: 10.1002/pbc.29217
Improving vitamin D testing and supplementation in children with newly diagnosed cancer: A quality improvement initiative at Rady Children's Hospital San Diego
Abstract
Background: Vitamin D deficiency and insufficiency have been associated with poorer health outcomes. Children with cancer are at high risk for vitamin D deficiency and insufficiency. At our institution, we identified high variability in vitamin D testing and supplementation in this population. Of those tested, 65% were vitamin D deficient/insufficient. We conducted a quality improvement (QI) initiative with aim to improve vitamin D testing and supplementation among children aged 2-18 years with newly diagnosed cancer to ≥80% over 6 months.
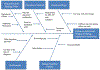
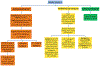
Methods: An inter-professional team reviewed baseline data, then developed and implemented interventions using Plan-Do-Study-Act (PDSA) cycles. Barriers were identified using QI tools, including lack of automated triggers for testing and inconsistent supplementation criteria and follow-up testing post supplementation. Interventions included an institutional vitamin D guideline, clinical decision-making tree for vitamin D deficiency, insufficiency and sufficiency, electronic medical record triggers, and automated testing options.
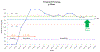
Results: Baseline: N = 26 patients, four (15%) had baseline vitamin D testing; two (8%) received appropriate supplementation. Postintervention: N = 33 patients; 32 (97%) had baseline vitamin D testing; 33 (100%) received appropriate supplementation and completed follow-up testing timely (6-8 weeks post supplementation). Change was sustained over 24 months.
Conclusions: We achieved and sustained our aim for vitamin D testing and supplementation in children with newly diagnosed cancer through inter-professional collaboration of hematology/oncology, endocrinology, hospital medicine, pharmacy, nursing, and information technology. Future PDSA cycles will address patient compliance with vitamin D supplementation and impact on patients' vitamin D levels.
Keywords: clinical guidelines; pediatric cancer; quality improvement; vitamin D deficiency; vitamin D supplementation.
© 2021 Wiley Periodicals LLC.
Figures



Similar articles
-
Sociodemographic and clinical characteristics associated with vitamin D status in newly diagnosed pediatric cancer patients.Pediatr Hematol Oncol. 2020 May;37(4):314-325. doi: 10.1080/08880018.2020.1721629. Epub 2020 Mar 10. Pediatr Hematol Oncol. 2020. PMID: 32153233 Free PMC article. Clinical Trial.
-
Vitamin D and Calcium Supplementation in Nursing Homes-A Quality Improvement Study.Nutrients. 2022 Dec 16;14(24):5360. doi: 10.3390/nu14245360. Nutrients. 2022. PMID: 36558519 Free PMC article.
-
Response of serum 25(OH)D to Vitamin D and calcium supplementation in school-children from a semi-rural setting in India.J Steroid Biochem Mol Biol. 2018 Jun;180:35-40. doi: 10.1016/j.jsbmb.2017.12.003. Epub 2017 Dec 13. J Steroid Biochem Mol Biol. 2018. PMID: 29247782 Clinical Trial.
-
Vitamin D supplementation for children with cancer: A systematic review and consensus recommendations.Cancer Med. 2021 Jul;10(13):4177-4194. doi: 10.1002/cam4.4013. Epub 2021 Jun 8. Cancer Med. 2021. PMID: 34100559 Free PMC article.
-
VItamin D supplementation in infants, children, and adolescents.Am Fam Physician. 2010 Mar 15;81(6):745-8. Am Fam Physician. 2010. PMID: 20229973 Review.
Cited by
-
Are Pediatric Cancer Patients a Risk Group for Vitamin D Deficiency? A Systematic Review.Cancers (Basel). 2024 Dec 17;16(24):4201. doi: 10.3390/cancers16244201. Cancers (Basel). 2024. PMID: 39766100 Free PMC article. Review.
-
Newly Diagnosed Children with Cancer Have Lower 25-Vitamin D Levels than Their Cancer-Free Peers: A Comparison across Age, Race, and Sex.Cancers (Basel). 2022 May 12;14(10):2378. doi: 10.3390/cancers14102378. Cancers (Basel). 2022. PMID: 35625982 Free PMC article.
References
-
- Medicine Io. Conclusions about Vitamin D deficiency in the US and Canada. 2011.

