Covalent and Noncovalent Targeting of the Tcf4/β-Catenin Strand Interface with β-Hairpin Mimics
- PMID: 34286954
- PMCID: PMC8687824
- DOI: 10.1021/acschembio.1c00389
Covalent and Noncovalent Targeting of the Tcf4/β-Catenin Strand Interface with β-Hairpin Mimics
Abstract
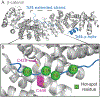
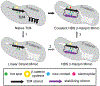
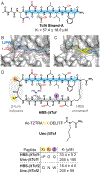
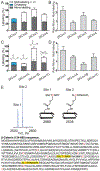
β-Strands are a fundamental component of protein structure, and these extended peptide regions serve as binding epitopes for numerous protein-protein complexes. However, synthetic mimics that capture the conformation of these epitopes and inhibit selected protein-protein interactions are rare. Here we describe covalent and noncovalent β-hairpin mimics of an extended strand region mediating the Tcf4/β-catenin interaction. Our efforts afford a rationally designed lead for an underexplored region of β-catenin, which has been the subject of numerous ligand discovery campaigns.
Figures

References
-
- Dou Y, Baisnee PF, Pollastri G, Pecout Y, Nowick J, and Baldi P (2004) ICBS: a database of interactions between protein chains mediated by -sheet formation, Bioinformatics 20, 2767–2777. - PubMed
-
- Guharoy M, and Chakrabarti P (2007) Secondary structure based analysis and classification of biological interfaces: identification of binding motifs in protein–protein interactions, Bioinformatics 23, 1909–1918. - PubMed
-
- Del Valle JR (2017) Heterocyclic Extended Peptide Surrogates for β-Strand Stabilization, in Peptidomimetics II (Lubell W, Ed.), pp 25–49, Springer International Publishing, Cham.
-
- Loughlin WA, Tyndall JDA, Glenn MP, and Fairlie DP (2004) Beta-Strand Mimetics, Chem. Rev 104, 6085–6118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

