Dental Plaque Microbial Resistomes of Periodontal Health and Disease and Their Changes after Scaling and Root Planing Therapy
- PMID: 34287005
- PMCID: PMC8386447
- DOI: 10.1128/mSphere.00162-21
Dental Plaque Microbial Resistomes of Periodontal Health and Disease and Their Changes after Scaling and Root Planing Therapy
Abstract
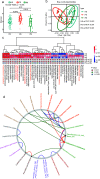
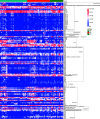
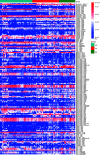
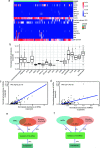
The human oral microbial community has been considered a reservoir of antibiotic resistance. Currently, the effects of periodontitis and the scaling and root planing (SRP) treatment on the performance of antibiotic-resistant genes (ARGs) and metal-resistant genes (MRGs) in the dental plaque microbiota are not well characterized. To explore this issue, we selected 48 healthy-state (HS), 40 periodontitis-state (PS; before treatment), and 24 resolved-state (RS; after SRP treatment) metagenomic data of dental plaque samples from the Sequence Read Archive (SRA) database. NetShift analysis identified Fretibacterium fastidiosum, Tannerella forsythia, and Campylobacter rectus as key drivers during dental plaque microbiota alteration in the progression of periodontitis. Periodontitis and SRP treatment resulted in an increase in the number of ARGs and MRGs in dental plaque and significantly altered the composition of ARG and MRG profiles. Bacitracin, beta-lactam, macrolide-lincosamide-streptogramin (MLS), tetracycline, and multidrug resistance genes were the main classes of ARGs with high relative abundance, whereas multimetal, iron, chromium, and copper resistance genes were the primary types of MRGs in dental plaque microbiota. The cooccurrence of ARGs, MRGs, and mobile genetic elements (MGEs) indicated that a coselection phenomenon exists in the resistomes of dental plaque microbiota. Overall, our data provide new insights into the standing of the distribution of ARGs and MRGs in oral microbiota of periodontitis patients, and it was possible to contribute to the understanding of the complicated correlations among microorganisms, resistomes, and MGEs. IMPORTANCE The emergence and development of resistance to antibiotics in periodontal pathogens have affected the success rate of treatment for periodontitis. The development of new antibacterial strategies is urgently needed to help control and treat periodontal disease, and dental plaque microbiome studies offer a promising new angle of attack. In this study, we investigated the dental plaque microbiota and resistomes in periodontal health and disease states and their changes after SRP therapy. This is the first analysis of the profile of the microbial community and antibiotic and metal resistance genes in dental plaque by the metagenomic approach, to the best of our knowledge. Monitoring the profile of these resistomes has huge potential to provide reference levels for proper antibiotics use and the development of new antimicrobial strategies in periodontitis therapy and thereby improve actual efficacy of the treatment regimens.
Keywords: antibiotic resistance genes; metagenomic analysis; metal resistance genes; microbial community; mobile genetic elements; periodontitis.
Figures


Similar articles
-
Metagenomic analysis of microbial community structure and distribution of resistance genes in Daihai Lake, China.Environ Pollut. 2022 Jun 1;302:119065. doi: 10.1016/j.envpol.2022.119065. Epub 2022 Feb 25. Environ Pollut. 2022. PMID: 35227842
-
Metagenomic Profiles of Yak and Cattle Manure Resistomes in Different Feeding Patterns before and after Composting.Appl Environ Microbiol. 2023 Jul 26;89(7):e0064523. doi: 10.1128/aem.00645-23. Epub 2023 Jul 6. Appl Environ Microbiol. 2023. PMID: 37409977 Free PMC article.
-
Metagenomic approach revealed the mobility and co-occurrence of antibiotic resistomes between non-intensive aquaculture environment and human.Microbiome. 2024 Jun 14;12(1):107. doi: 10.1186/s40168-024-01824-x. Microbiome. 2024. PMID: 38877573 Free PMC article.
-
Pharmacologic management of periodontal diseases using systemically administered agents.Dent Clin North Am. 1998 Apr;42(2):245-62. Dent Clin North Am. 1998. PMID: 9597336 Review.
-
Microbial colonization of the periodontal pocket and its significance for periodontal therapy.Periodontol 2000. 2018 Feb;76(1):85-96. doi: 10.1111/prd.12147. Epub 2017 Nov 30. Periodontol 2000. 2018. PMID: 29193304 Review.
Cited by
-
Managing Oral Health in the Context of Antimicrobial Resistance.Int J Environ Res Public Health. 2022 Dec 8;19(24):16448. doi: 10.3390/ijerph192416448. Int J Environ Res Public Health. 2022. PMID: 36554332 Free PMC article. Review.
-
Immunomodulators and Their Applications in Dentistry and Periodontics: A Comprehensive Review.Cureus. 2023 Oct 7;15(10):e46653. doi: 10.7759/cureus.46653. eCollection 2023 Oct. Cureus. 2023. PMID: 37937011 Free PMC article. Review.
-
Antibiotic Resistance in Patients with Peri-Implantitis: A Systematic Scoping Review.Int J Environ Res Public Health. 2022 Nov 24;19(23):15609. doi: 10.3390/ijerph192315609. Int J Environ Res Public Health. 2022. PMID: 36497685 Free PMC article.
-
Unveiling the Resistome Landscape in Peri-Implant Health and Disease.J Clin Med. 2025 Jan 31;14(3):931. doi: 10.3390/jcm14030931. J Clin Med. 2025. PMID: 39941602 Free PMC article.
-
Effect of Gum Arabic on plaque-induced gingivitis: A randomised controlled trial.Saudi Dent J. 2022 Sep;34(6):494-502. doi: 10.1016/j.sdentj.2022.06.002. Epub 2022 Jun 13. Saudi Dent J. 2022. PMID: 36092515 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
