Application of an in Vitro Assay to Identify Chemicals That Increase Estradiol and Progesterone Synthesis and Are Potential Breast Cancer Risk Factors
- PMID: 34287026
- PMCID: PMC8293912
- DOI: 10.1289/EHP8608
Application of an in Vitro Assay to Identify Chemicals That Increase Estradiol and Progesterone Synthesis and Are Potential Breast Cancer Risk Factors
Abstract
Background: Established breast cancer risk factors, such as hormone replacement therapy and reproductive history, are thought to act by increasing estrogen and progesterone (P4) activity.
Objective: We aimed to use in vitro screening data to identify chemicals that increase the synthesis of estradiol (E2) or P4 and evaluate potential risks.
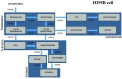
Method: Using data from a high-throughput (HT) in vitro steroidogenesis assay developed for the U.S. Environmental Protection Agency (EPA) ToxCast program, we identified chemicals that increased estradiol (E2-up) or progesterone (P4-up) in human H295R adrenocortical carcinoma cells. We prioritized chemicals by their activity. We compiled in vivo studies and assessments about carcinogenicity and reproductive/developmental (repro/dev) toxicity. We identified exposure sources and predicted intakes from the U.S. EPA's ExpoCast.
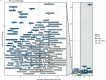
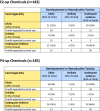
Results: We found 296 chemicals increased E2 (182) or P4 (185), with 71 chemicals increasing both. In vivo data often showed effects consistent with this mechanism. Of the E2- and P4-up chemicals, about 30% were likely repro/dev toxicants or carcinogens, whereas only 5-13% were classified as unlikely. However, most of the chemicals had insufficient in vivo data to evaluate their effects. Of 45 chemicals associated with mammary gland effects, and also tested in the H294R assay, 29 increased E2 or P4, including the well-known mammary carcinogen 7,12-dimethylbenz(a)anthracene. E2- and P4-up chemicals include pesticides, consumer product ingredients, food additives, and drinking water contaminants.
Discussion: The U.S. EPA's in vitro screening data identified several hundred chemicals that should be considered as potential risk factors for breast cancer because they increased E2 or P4 synthesis. In vitro data is a helpful addition to current toxicity assessments, which are not sensitive to mammary gland effects. Relevant effects on the mammary gland are often not noticed or are dismissed, including for 2,4-dichlorophenol and cyfluthrin. Fifty-three active E2-up and 59 active P4-up chemicals that are in consumer products, food, pesticides, or drugs have not been evaluated for carcinogenic potential and are priorities for study and exposure reduction. https://doi.org/10.1289/EHP8608.
Figures

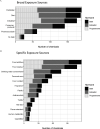
![Figure 5 is set of three graphs plotting Chemical name, namely, from bottom to top, 2-hydroxy-4-methoxybenzophenone, 2,6-Dinitrotulene, 4-Nitrophenol, Cycloate, Dicyclohexylamine, 3,4-Dicholoroaniline, 3,4-Dimethylphenol; 3,4-Dinitrotluene; 4-Chloro-2-methyllaniline, Acibenzolar-S-methyl, 2-Naphthalenol, 2,3-Dinitrotulene, Molinate, Bisphenol A, Methylparaben, 4-Methoxyaniline hydrochloride, 5,7-Dimethoxy-2H-chromen-2-one, Azobenzene, Propylparaben, 4-(2-Methylbutan-2-yl)phenol, 2-(3-Phenylpropyl)pyridine, 3, 3 prime Dimethoxybenzidine dihydrochloride, 3,3,5-Trimethylcyclohexyl salicylate, 3-Methyl-4-(methylthio)phenol, Resorcinol, Catechol, 1,3-Bis(4-aminophenoxy) benzene, 4-Chloro-3-methylphenol, Diphenylamine, 3,3 prime, 5, 5 prime-Tetrabromobisphenol A, 4-Butylphenol, 2-Hydroxyethyl acrylate, Hydroquinone, Methyl dihydrojasmonate, 2,4-Dimethylphenol, 4,4 prime-Sulfonylbis[2-(prop-2-en-1-yl)phenol], N,N-Dimethyldoctylamine, Acid Orange, Methyleugenol, 2-Methyl-5-nitroaniline, Di(2-ethylhexyl) phthalate, 4-(Butan-2-yl)phenol, 3-tert-Butylphenol, 2,5-Dichlorophenol, 2,4-Dichlorophenol, Diphenyl isophthalate, 2, 4, 6-Tribromophenol, 2-Methoxy-5-methyaniline, p-Cresol, Dimethyl isophthalate, 10-Undecenoic acid, Methyl 2,4-dihydroxy-3,6-dimethylbenzoate, Nitrilotriacetic acid, and 3-Isopropylphenol (y-axis) across median exposure rate (milligrams per kilogram body weight per day), ranging from 0.00 to 0.25 in increments of 0.05, 0.5 to 2.0 in increments of 0.5, and 5 to 55 in increments of 10 (x-axis) for efficacy or potency, namely, lower, intermediate, and higher and Hormone, namely, both, estradiol, and progesterone, respectively.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ad6d/8293912/1d7d787d72d8/ehp8608_f5.gif)
Comment in
-
Invited Perspective: Prioritizing Chemical Testing and Evaluation Using Validated in Vitro Assays Relevant to Key Characteristics.Environ Health Perspect. 2021 Jul;129(7):71303. doi: 10.1289/EHP9507. Epub 2021 Jul 21. Environ Health Perspect. 2021. PMID: 34287027 Free PMC article. No abstract available.
-
Response to "Comment on 'Application of an in Vitro Assay to Identify Chemicals That Increase Estradiol and Progesterone Synthesis and Are Potential Breast Cancer Risk Factors'".Environ Health Perspect. 2022 May;130(5):58003. doi: 10.1289/EHP11400. Epub 2022 May 4. Environ Health Perspect. 2022. PMID: 35507338 Free PMC article. No abstract available.
-
Comment on "Application of an in Vitro Assay to Identify Chemicals That Increase Estradiol and Progesterone Synthesis and Are Potential Breast Cancer Risk Factors".Environ Health Perspect. 2022 May;130(5):58002. doi: 10.1289/EHP11083. Epub 2022 May 4. Environ Health Perspect. 2022. PMID: 35507340 Free PMC article. No abstract available.
References
-
- Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER. 1996. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. J Clin Endocrinol Metab 81(11):3843–3849, PMID: 8923826, 10.1210/jcem.81.11.8923826. - DOI - PubMed
-
- Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Calafat AM, et al. . 2019. A repeated measures study of phenol, paraben and Triclocarban urinary biomarkers and circulating maternal hormones during gestation in the Puerto Rico PROTECT cohort. Environ Health 18(1):28, PMID: 30940137, 10.1186/s12940-019-0459-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

