Delayed cytokine release syndrome after neoadjuvant nivolumab: a case report and literature review
- PMID: 34287029
- PMCID: PMC8656293
- DOI: 10.2217/imt-2020-0329
Delayed cytokine release syndrome after neoadjuvant nivolumab: a case report and literature review
Abstract
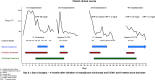
Aim: Cytokine release syndrome (CRS) is an infrequently described immune-related adverse event of checkpoint inhibitors (CPI). CPI-induced CRS typically presents with fevers, hemodynamic instability and organ dysfunction within 2 weeks of the last treatment cycle. Case study: We report an unusual case of delayed and severe CRS occurring postoperatively in a patient with hepatic-limited metastatic colorectal cancer who received neoadjuvant immunotherapy. After a negative workup for alternative causes, he received prolonged corticosteroid treatment with symptom resolution. Conclusion: CPI-induced CRS can mimic sepsis and clinicians should maintain a high-index of suspicion to diagnose this immune-related adverse event early and initiate appropriate treatment. As use of perioperative immunotherapy increases, the potential role of surgery to trigger CRS in this case warrants further investigation.
Keywords: colorectal cancer; cytokine release syndrome; immunotherapy; neoadjuvant treatment; surgical-induced inflammation.
Plain language summary
Lay abstract Aim: Cytokine release syndrome (CRS) is a rare but potentially serious side effect of a class of immunotherapy drugs called checkpoint inhibitors (CPI). CRS typically presents with fevers and low blood pressure and can cause damage to organs including the kidneys and liver. Case study: We report an unusual case of severe CRS occurring after surgery in a patient who had received prior CPI therapy. After a thorough evaluation for alternative causes, he was diagnosed with CRS and treated successfully with steroids. Conclusion: It is important for medical providers to consider this potential side effect when treating patients with CPI. Further research is needed to clarify the role of surgery in CPI-induced CRS.
Conflict of interest statement
D Carpizo is NIH funded. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
Similar articles
-
Tocilizumab and CytoSorb for delayed severe cytokine release syndrome after ipilimumab plus nivolumab immunotherapy.Immunotherapy. 2024;16(12):791-801. doi: 10.1080/1750743X.2024.2370180. Epub 2024 Jul 17. Immunotherapy. 2024. PMID: 39016056 Free PMC article.
-
Cytokine Release Syndrome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Case Series of 25 Patients and Review of the Literature.Front Immunol. 2022 Jan 28;13:807050. doi: 10.3389/fimmu.2022.807050. eCollection 2022. Front Immunol. 2022. PMID: 35154124 Free PMC article. Review.
-
Severe Cytokine Release Syndrome and Immune Effector Cell-associated Neurotoxicity Syndrome in a Man Receiving Immune Checkpoint Inhibitors for Lung Cancer.Intern Med. 2024 May 1;63(9):1261-1267. doi: 10.2169/internalmedicine.2429-23. Epub 2023 Sep 15. Intern Med. 2024. PMID: 37722894 Free PMC article.
-
Nivolumab-Induced Cytokine Release Syndrome: A Case Report and Literature Review.Am J Case Rep. 2024 Apr 16;25:e941835. doi: 10.12659/AJCR.941835. Am J Case Rep. 2024. PMID: 38625840 Free PMC article. Review.
-
Successful Application of Tocilizumab in a Patient With Neoadjuvant Immunochemotherapy-Induced Cytokine Release Syndrome.Cancer Rep (Hoboken). 2024 Jul;7(7):e2145. doi: 10.1002/cnr2.2145. Cancer Rep (Hoboken). 2024. PMID: 39051558 Free PMC article.
Cited by
-
Tocilizumab and CytoSorb for delayed severe cytokine release syndrome after ipilimumab plus nivolumab immunotherapy.Immunotherapy. 2024;16(12):791-801. doi: 10.1080/1750743X.2024.2370180. Epub 2024 Jul 17. Immunotherapy. 2024. PMID: 39016056 Free PMC article.
-
Innovative CDR grafting and computational methods for PD-1 specific nanobody design.Front Bioinform. 2025 Jan 17;4:1488331. doi: 10.3389/fbinf.2024.1488331. eCollection 2024. Front Bioinform. 2025. PMID: 39897125 Free PMC article.
-
Case report: Savolitinib induced severe adverse reactions resembling septic shock in an HIV-1-positive patient with advanced non-small cell lung cancer.Front Pharmacol. 2023 Feb 2;14:1089184. doi: 10.3389/fphar.2023.1089184. eCollection 2023. Front Pharmacol. 2023. PMID: 36817157 Free PMC article.
-
Herb pair of Huangqi-Danggui exerts anti-tumor immunity to breast cancer by upregulating PIK3R1.Animal Model Exp Med. 2024 Jun;7(3):234-258. doi: 10.1002/ame2.12434. Epub 2024 Jun 11. Animal Model Exp Med. 2024. PMID: 38863309 Free PMC article.
-
Immunotherapy for Triple-Negative Breast Cancer: Combination Strategies to Improve Outcome.Cancers (Basel). 2023 Jan 3;15(1):321. doi: 10.3390/cancers15010321. Cancers (Basel). 2023. PMID: 36612317 Free PMC article. Review.
References
-
- Queirolo P, Boutros A, Tanda E, Spagnolo F, Quaglino P. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: a model of cancer immunotherapy. Semin. Cancer Biol. 59, 290–297 (2019). - PubMed
-
- Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer. Nat. Rev. Urol. 17(3), 137–150 (2020). - PubMed
-
- Wilky BA. Immune checkpoint inhibitors: the linchpins of modern immunotherapy. Immunol. Rev. 290(1), 6–23 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials