Investigating the Roles of Listeria monocytogenes Peroxidases in Growth and Virulence
- PMID: 34287055
- PMCID: PMC8552690
- DOI: 10.1128/Spectrum.00440-21
Investigating the Roles of Listeria monocytogenes Peroxidases in Growth and Virulence
Abstract
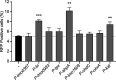
Bacteria have necessarily evolved a protective arsenal of proteins to contend with peroxides and other reactive oxygen species generated in aerobic environments. Listeria monocytogenes encounters an onslaught of peroxide both in the environment and during infection of the mammalian host, where it is the causative agent of the foodborne illness listeriosis. Despite the importance of peroxide for the immune response to bacterial infection, the strategy by which L. monocytogenes protects against peroxide toxicity has yet to be illuminated. Here, we investigated the expression and essentiality of all the peroxidase-encoding genes during L. monocytogenes growth in vitro and during infection of murine cells in tissue culture. We found that chdC and kat were required for aerobic growth in vitro, and fri and ahpA were each required for L. monocytogenes to survive acute peroxide stress. Despite increased expression of fri, ahpA, and kat during infection of macrophages, only fri proved necessary for cytosolic growth. In contrast, the proteins encoded by lmo0367, lmo0983, tpx, lmo1609, and ohrA were dispensable for aerobic growth, acute peroxide detoxification, and infection. Together, our results provide insight into the multifaceted L. monocytogenes peroxide detoxification strategy and demonstrate that L. monocytogenes encodes a functionally diverse set of peroxidase enzymes. IMPORTANCE Listeria monocytogenes is a facultative intracellular pathogen and the causative agent of the foodborne illness listeriosis. L. monocytogenes must contend with reactive oxygen species generated extracellularly during aerobic growth and intracellularly by the host immune system. However, the mechanisms by which L. monocytogenes defends against peroxide toxicity have not yet been defined. Here, we investigated the roles of each of the peroxidase-encoding genes in L. monocytogenes growth, peroxide stress response, and virulence in mammalian cells.
Keywords: heme; oxidative stress; peroxide; redox signaling; virulence.
Figures




References
-
- Nauseef WM, Clark RA. 2019. Intersecting stories of the phagocyte NADPH oxidase and chronic granulomatous disease, p 3–16. In Knaus UG, Leto TL (ed), NADPH oxidases: methods and protocols. Springer, New York, NY. - PubMed
-
- van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Español T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia M-J, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. 2009. Chronic granulomatous disease: the European experience. PLoS One 4:e5234. doi:10.1371/journal.pone.0005234. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

