Drosophila Corazonin Neurons as a Hub for Regulating Growth, Stress Responses, Ethanol-Related Behaviors, Copulation Persistence and Sexually Dimorphic Reward Pathways
- PMID: 34287347
- PMCID: PMC8293205
- DOI: 10.3390/jdb9030026
Drosophila Corazonin Neurons as a Hub for Regulating Growth, Stress Responses, Ethanol-Related Behaviors, Copulation Persistence and Sexually Dimorphic Reward Pathways
Abstract
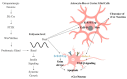
The neuronal mechanisms by which complex behaviors are coordinated and timed often involve neuropeptidergic regulation of stress and reward pathways. Recent studies of the neuropeptide Corazonin (Crz), a homolog of the mammalian Gonadotrophin Releasing Hormone (GnRH), have suggested its crucial role in the regulation of growth, internal states and behavioral decision making. We focus this review on Crz neurons with the goal to (1) highlight the diverse roles of Crz neuron function, including mechanisms that may be independent of the Crz peptide, (2) emphasize current gaps in knowledge about Crz neuron functions, and (3) propose exciting ideas of novel research directions involving the use of Crz neurons. We describe the different developmental fates of distinct subsets of Crz neurons, including recent findings elucidating the molecular regulation of apoptosis. Crz regulates systemic growth, food intake, stress responses and homeostasis by interacting with the short Neuropeptide F (sNPF) and the steroid hormone ecdysone. Additionally, activation of Crz neurons is shown to be pleasurable by interacting with the Neuropeptide F (NPF) and regulates reward processes such as ejaculation and ethanol-related behaviors in a sexually dimorphic manner. Crz neurons are proposed to be a motivational switch regulating copulation duration using a CaMKII-dependent mechanism described as the first neuronal interval timer lasting longer than a few seconds. Lastly, we propose ideas to use Crz neuron-induced ejaculation to study the effects of fictive mating and sex addiction in flies, as well as to elucidate dimorphic molecular mechanisms underlying reward behaviors and feeding disorders.
Keywords: addiction; alcoholism; dimorphism; eating disorder; growth; insect disease model; neuropeptide.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Ejaculation Induced by the Activation of Crz Neurons Is Rewarding to Drosophila Males.Curr Biol. 2018 May 7;28(9):1445-1452.e3. doi: 10.1016/j.cub.2018.03.039. Epub 2018 Apr 19. Curr Biol. 2018. PMID: 29681474
-
Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin.Cell Mol Life Sci. 2012 Dec;69(23):4051-66. doi: 10.1007/s00018-012-1097-z. Epub 2012 Jul 25. Cell Mol Life Sci. 2012. PMID: 22828865 Free PMC article.
-
The Corazonin-PTTH Neuronal Axis Controls Systemic Body Growth by Regulating Basal Ecdysteroid Biosynthesis in Drosophila melanogaster.Curr Biol. 2020 Jun 8;30(11):2156-2165.e5. doi: 10.1016/j.cub.2020.03.050. Epub 2020 May 7. Curr Biol. 2020. PMID: 32386525
-
GnRH-Related Neurohormones in the Fruit Fly Drosophila melanogaster.Int J Mol Sci. 2021 May 10;22(9):5035. doi: 10.3390/ijms22095035. Int J Mol Sci. 2021. PMID: 34068603 Free PMC article. Review.
-
In search for a common denominator for the diverse functions of arthropod corazonin: a role in the physiology of stress?Gen Comp Endocrinol. 2010 Apr 1;166(2):222-33. doi: 10.1016/j.ygcen.2009.09.004. Epub 2009 Sep 11. Gen Comp Endocrinol. 2010. PMID: 19748506 Review.
Cited by
-
Neurons for Ejaculation and Factors Affecting Ejaculation.Biology (Basel). 2022 Apr 29;11(5):686. doi: 10.3390/biology11050686. Biology (Basel). 2022. PMID: 35625414 Free PMC article. Review.
-
Drosophila melanogaster as a neurobehavioral model for sex differences in stress response.Front Behav Neurosci. 2025 Jul 11;19:1581763. doi: 10.3389/fnbeh.2025.1581763. eCollection 2025. Front Behav Neurosci. 2025. PMID: 40718064 Free PMC article. Review.
-
Food supplementation with wheat gluten leads to climbing performance decline in Drosophila melanogaster.MicroPubl Biol. 2022 Sep 23;2022:10.17912/micropub.biology.000642. doi: 10.17912/micropub.biology.000642. eCollection 2022. MicroPubl Biol. 2022. PMID: 36217442 Free PMC article.
-
A Brief Overview of Ethanol Tolerance and Its Potential Association with Circadian Rhythm in Drosophila.Int J Mol Sci. 2024 Nov 24;25(23):12605. doi: 10.3390/ijms252312605. Int J Mol Sci. 2024. PMID: 39684317 Free PMC article. Review.
-
Exposure to female olfactory cues hastens reproductive ageing and increases mortality when mating in male mice.Proc Biol Sci. 2024 Feb 28;291(2017):20231848. doi: 10.1098/rspb.2023.1848. Epub 2024 Feb 28. Proc Biol Sci. 2024. PMID: 38412966 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

