Chondroitin sulfate from fish waste exhibits strong intracellular antioxidant potential
- PMID: 34287577
- PMCID: PMC8289345
- DOI: 10.1590/1414-431X2020e10730
Chondroitin sulfate from fish waste exhibits strong intracellular antioxidant potential
Abstract
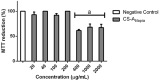
Chondroitin sulfate (CS) is a type of glycosaminoglycan described as an antioxidant molecule that has been found in animal species such as fish. Tilapia (Oreochromis niloticus) represents an eco-friendly source of this compound, since its economical processing generates usable waste, reducing the negative environmental impact. This waste was used for CS extraction, purification, characterization by enzymatic degradation, and evaluation of its antioxidant effect. CS obtained from tilapia presented sulfation mainly at carbon 4 of galactosamine, and it was not cytotoxic at concentrations up to 200 µg/mL. Furthermore, 100 µg/mL of CS from tilapia reduced the levels of reactive oxygen species to 47% of the total intracellular reactive oxygen species level. The ability of CS to chelate metal ions in vitro also suggested an ability to react with other pathways that generate oxidative radicals, such as the Haber-Weiss reaction, acting intracellularly in more than one way. Although the role of CS from tilapia remains unclear, the pharmacological effects described herein indicate that CS is a potential molecule for further study of the relationship between the structures and functions of chondroitin sulfates as antioxidants.
Figures


Similar articles
-
Chondroitin Sulfate from Oreochromis niloticus Waste Reduces Leukocyte Influx in an Acute Peritonitis Model.Molecules. 2023 Mar 30;28(7):3082. doi: 10.3390/molecules28073082. Molecules. 2023. PMID: 37049845 Free PMC article.
-
Obtaining glycosaminoglycans from tilapia (oreochromis niloticus) scales and evaluation of its anticoagulant and cytotoxic activities: Glycosaminoglycans from tilapia scales: anticoagulant and cytotoxic activities.Food Res Int. 2021 Feb;140:110012. doi: 10.1016/j.foodres.2020.110012. Epub 2020 Dec 15. Food Res Int. 2021. PMID: 33648244
-
Viscera of fishes as raw material for extraction of glycosaminoglycans of pharmacological interest.Int J Biol Macromol. 2019 Jan;121:239-248. doi: 10.1016/j.ijbiomac.2018.09.156. Epub 2018 Sep 26. Int J Biol Macromol. 2019. PMID: 30267823
-
The dual role of the glycosaminoglycan chondroitin-6-sulfate in the development, progression and metastasis of cancer.FEBS J. 2019 May;286(10):1815-1837. doi: 10.1111/febs.14748. Epub 2019 Feb 5. FEBS J. 2019. PMID: 30637950 Free PMC article. Review.
-
Biosynthesis and function of chondroitin sulfate.Biochim Biophys Acta. 2013 Oct;1830(10):4719-33. doi: 10.1016/j.bbagen.2013.06.006. Epub 2013 Jun 14. Biochim Biophys Acta. 2013. PMID: 23774590 Review.
Cited by
-
Chondroitin Sulfate from Oreochromis niloticus Waste Reduces Leukocyte Influx in an Acute Peritonitis Model.Molecules. 2023 Mar 30;28(7):3082. doi: 10.3390/molecules28073082. Molecules. 2023. PMID: 37049845 Free PMC article.
-
Exploring the mechanism of chondroitin sulfate-selenium nanoparticles in improving Alzheimer's disease: Insights from intestinal flora evaluation.Heliyon. 2024 Sep 28;10(19):e38635. doi: 10.1016/j.heliyon.2024.e38635. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39421360 Free PMC article.
-
A Concise Review of Extraction and Characterization of Chondroitin Sulphate from Fish and Fish Wastes for Pharmacological Application.Curr Issues Mol Biol. 2022 Aug 28;44(9):3905-3922. doi: 10.3390/cimb44090268. Curr Issues Mol Biol. 2022. PMID: 36135180 Free PMC article. Review.
-
Tilapia-Head Chondroitin Sulfate Protects against Nonalcoholic Fatty Liver Disease via Modulating the Gut-Liver Axis in High-Fat-Diet-Fed C57BL/6 Mice.Foods. 2022 Mar 23;11(7):922. doi: 10.3390/foods11070922. Foods. 2022. PMID: 35407014 Free PMC article.
-
Efficacy and Safety Assessment of a Dietary Supplement in a Rat Model of Osteoarthritis and Dogs with Arthritic Signs.Animals (Basel). 2025 Jun 20;15(13):1825. doi: 10.3390/ani15131825. Animals (Basel). 2025. PMID: 40646726 Free PMC article.
References
-
- Uyama T, Kitagawa H, Sugahara K. Biosynthesis of glycosaminoglycans and proteoglycans. Comprehensive Glycosci. 2007;3:79–104. doi: 10.1016/B978-044451967-2/00036-2. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

