Competitive blocking of salivary gland [18F]DCFPyL uptake via localized, retrograde ductal injection of non-radioactive DCFPyL: a preclinical study
- PMID: 34287731
- PMCID: PMC8295433
- DOI: 10.1186/s13550-021-00803-9
Competitive blocking of salivary gland [18F]DCFPyL uptake via localized, retrograde ductal injection of non-radioactive DCFPyL: a preclinical study
Abstract
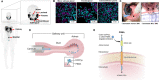
Background: PSMA-targeted radionuclide therapy (TRT) is a promising treatment for prostate cancer (PCa), but dose-limiting xerostomia can severely limit its clinical adaptation, especially when using alpha-emitting radionuclides. With [18F]DCFPyL as a surrogate for PSMA-TRT, we report a novel method to selectively reduce salivary gland (SG) uptake of systemically administered [18F]DCFPyL by immediate prior infusion of non-radioactive standard of [18F]DCFPyL (DCFPyL) directly into the SG via retrograde cannulation.
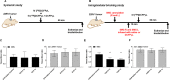
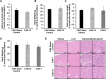
Methods: A dose-finding cohort using athymic nude mice demonstrated proof of principle that SG uptake can be selectively blocked by DCFPyL administered either locally via cannulation (CAN group) or systemically (SYS group). The experiments were repeated in a validation cohort of 22RV1 tumor-bearing mice. Submandibular glands (SMG) of CAN mice were locally blocked with either saline or DCFPyL (dose range: 0.01× to 1000× molar equivalent of the radioactive [18F]DCFPyL dose). The radioactive dose of [18F]DCFPyL was administered systemically 10 min later and the mice euthanized after 1 h for biodistribution studies. Toxicity studies were done at up to 1000× dose.
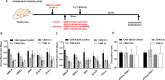
Results: In the dose-finding cohort, the SYS group showed a dose-dependent 12-40% decrease in both the SMG T/B and the kidney (tumor surrogate). Mild blocking was observed at 0.01× , with maximal blocking reached at 1× with no additional blocking up to 1000× . In the CAN group, blocking at the 0.1× and 1× dose levels resulted in a similar 42-53% decrease, but without the corresponding decrease in kidney uptake as seen in the SYS group. Some evidence of "leakage" of DCFPyL from the salivary gland into the systemic circulation was observed. However, experiments in 22RV1 tumor-bearing mice at the 0.1× and 1× dose levels confirm that, at the appropriate blocking dose, SG uptake of [18F]DCFPyL can be selectively reduced without affecting tumor uptake and with no toxicity.
Conclusion: Our results suggest that direct retrograde instillation of DCFPyL into the SG could predictably and selectively decrease salivary uptake of systemically administered [18F]DCFPyL without altering tumor uptake, if given at the appropriate dose. This novel approach is easily translatable to clinical practice and has the potential to mitigate xerostomia, without compromising the therapeutic efficacy of the PSMA-TRT.
Keywords: Cannulation; Competitive inhibition; PSMA; Prostate cancer; Radionuclide therapy; Salivary glands; Xerostomia.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

