Abdominal incision defect following AAA-surgery (AIDA): 2-year results of prophylactic onlay-mesh augmentation in a multicentre, double-blind, randomised controlled trial
- PMID: 34287760
- PMCID: PMC9213335
- DOI: 10.1007/s13304-021-01125-0
Abdominal incision defect following AAA-surgery (AIDA): 2-year results of prophylactic onlay-mesh augmentation in a multicentre, double-blind, randomised controlled trial
Abstract
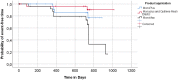
The reported incidence of incisional hernia following repair of abdominal aortic aneurysm (AAA) via midline laparotomy is up to 69%. This prospective, multicenter, double-blind, randomised controlled trial was conducted at eleven hospitals in Germany. Patients aged 18 years or older undergoing elective AAA-repair via midline incision were randomly assigned using a computer-generated randomisation sequence to one of three groups for fascial closure: with long-term absorbable suture (MonoPlus®, group I), long-term absorbable suture and onlay mesh reinforcement (group II) or extra long-term absorbable suture (MonoMax®, group III). The primary endpoint was the incidence of incisional hernia within 24 months of follow-up, analysed by intention to treat. Physicians conducting the postoperative visits and the patients were blinded. Between February 2011 and July 2013, 104 patients (69.8 ± 7.7 years) were randomised, 99 of them received a study intervention. The rate of incisional hernia within 24 months was not significantly reduced with onlay mesh augmentation compared to primary suture (p = 0.290). Furthermore, the rate of incisional hernia did not differ significantly between fascial closure with slow and extra long-term absorbable suture (p = 0.111). Serious adverse events related to study intervention occurred in five patients (5.1%) from treatment groups II and III. Wound healing disorders were more frequently seen after onlay mesh implantation on the day of discharge (p = 0.010) and three (p = 0.009) and six (p = 0.023) months postoperatively. The existing evidence on prophylactic mesh augmentation in patients undergoing AAA-repair via midline laparotomy probably needs critical review. As the implementation of new RCTs is considered difficult due to the increasing number of endovascular AAA treated, registry studies could help to collect and evaluate data in cases of open AAA-repair. Comparisons between prophylactic mesh implantation and the small bite technique are also required. Trial registration: ClinicalTrials.gov Identifier: NCT01353443. Funding Sources: Aesculap AG, Tuttlingen, Germany.
Keywords: Abdominal; Aortic aneurysm; Fascial suture closure; Incisional hernia; Onlay mesh; Randomised controlled trial.
© 2021. The Author(s).
Conflict of interest statement
We declare no competing interests.
Figures

Comment in
-
Prevention of incisional hernia after midline laparotomy for abdominal aortic aneurysm repair.Updates Surg. 2022 Jun;74(3):1173-1174. doi: 10.1007/s13304-021-01164-7. Epub 2021 Sep 4. Updates Surg. 2022. PMID: 34480731 No abstract available.
-
Too Limited Use of Prophylactic Mesh After Open AAA Repair in Belgium and The Netherlands?Eur J Vasc Endovasc Surg. 2022 May;63(5):775-776. doi: 10.1016/j.ejvs.2022.02.031. Epub 2022 Feb 24. Eur J Vasc Endovasc Surg. 2022. PMID: 35393242 No abstract available.
Similar articles
-
Prevention of incisional hernia with prophylactic onlay and sublay mesh reinforcement versus primary suture only in midline laparotomies (PRIMA): 2-year follow-up of a multicentre, double-blind, randomised controlled trial.Lancet. 2017 Aug 5;390(10094):567-576. doi: 10.1016/S0140-6736(17)31332-6. Epub 2017 Jun 20. Lancet. 2017. PMID: 28641875 Clinical Trial.
-
Hernia reduction following laparotomy using small stitch abdominal wall closure with and without mesh augmentation (the HULC trial): study protocol for a randomized controlled trial.Trials. 2019 Dec 16;20(1):738. doi: 10.1186/s13063-019-3921-3. Trials. 2019. PMID: 31842966 Free PMC article.
-
5-year clinical outcome of the ESTOIH trial comparing the short-bite versus large-bite technique for elective midline abdominal closure.Hernia. 2025 Aug 29;29(1):263. doi: 10.1007/s10029-025-03459-9. Hernia. 2025. PMID: 40879826 Free PMC article. Clinical Trial.
-
Prophylactic Mesh Reinforcement versus Sutured Closure to Prevent Incisional Hernias after Open Abdominal Aortic Aneurysm Repair via Midline Laparotomy: A Systematic Review and Meta-Analysis.Eur J Vasc Endovasc Surg. 2018 Jul;56(1):120-128. doi: 10.1016/j.ejvs.2018.03.021. Epub 2018 Apr 22. Eur J Vasc Endovasc Surg. 2018. PMID: 29685678
-
Does prophylactic mesh placement in elective, midline laparotomy reduce the incidence of incisional hernia? A systematic review and meta-analysis.Surgery. 2017 Apr;161(4):1149-1163. doi: 10.1016/j.surg.2016.09.036. Epub 2016 Dec 28. Surgery. 2017. PMID: 28040255
Cited by
-
Prevention of incisional hernia after midline laparotomy for abdominal aortic aneurysm repair.Updates Surg. 2022 Jun;74(3):1173-1174. doi: 10.1007/s13304-021-01164-7. Epub 2021 Sep 4. Updates Surg. 2022. PMID: 34480731 No abstract available.
-
Mapping the therapeutic landscape in emergency incisional hernia: a scoping review.Hernia. 2025 Feb 18;29(1):102. doi: 10.1007/s10029-025-03278-y. Hernia. 2025. PMID: 39966185 Free PMC article.
-
Updated guideline for closure of abdominal wall incisions from the European and American Hernia Societies.Br J Surg. 2022 Nov 22;109(12):1239-1250. doi: 10.1093/bjs/znac302. Br J Surg. 2022. PMID: 36026550 Free PMC article.
-
Incisional Hernias after Vascular Surgery for Aortoiliac Aneurysm and Aortoiliac Occlusive Arterial Disease: Has Prophylactic Mesh Changed This Scenario?Aorta (Stamford). 2023 Jun;11(3):107-111. doi: 10.1055/s-0043-1771475. Epub 2023 Aug 24. Aorta (Stamford). 2023. PMID: 37619567 Free PMC article.
-
Prophylactic mesh versus primary closure in emergency and elective surgeries: a systematic review and meta-analysis of randomized clinical trials.Hernia. 2024 Nov 16;29(1):14. doi: 10.1007/s10029-024-03202-w. Hernia. 2024. PMID: 39549074
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

