Bioavailability of a Capsaicin Lipid Multi-particulate Formulation in Rats
- PMID: 34287807
- PMCID: PMC8397674
- DOI: 10.1007/s13318-021-00697-x
Bioavailability of a Capsaicin Lipid Multi-particulate Formulation in Rats
Erratum in
-
Correction to: Bioavailability of a Capsaicin Lipid Multi‑particulate Formulation in Rats.Eur J Drug Metab Pharmacokinet. 2021 Sep;46(5):651. doi: 10.1007/s13318-021-00710-3. Eur J Drug Metab Pharmacokinet. 2021. PMID: 34420203 Free PMC article. No abstract available.
Abstract
Background and objective: Because of the stomach-burning sensation it induces, capsaicin has been used at relatively low doses as a nutritional supplement, which has limited its bioavailability. The objective of this study was to investigate the serum bioavailability of capsaicin supplementation with or without a lipid multi-particulate (LMP) formulation.
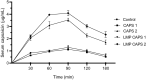
Methods: Thirty-five rats were divided into five groups and administered capsaicin at either 0.2 or 1 mg/kg with or without the LMP formulation. Capsaicin bioavailability was assessed based on the area under the concentation-time curve (AUC), the time to peak concentration (Tmax), and the peak serum concentration (Cmax).
Results: For each formulation, the capsaicin Cmax was reached at 90 min and decreased thereafter. Serum capsaicin concentrations were greater in rats administered the higher dose of capsaicin (1 mg/kg) in the LMP formulation at all measurement times (P ≤ 0.05). The AUC showed a significant increase, about 20%, when capsaicin was administered in the LMP formulation at the high dose (P = 0.002). The Tmax for oral capsaicin was similar whether or not administration was via the LMP formulation (P = 0.163). However, the Cmax of capsaicin increased in a dose-dependent manner (P < 0.05). Although the LMP formulation of the high dose of capsaicin resulted in a numerically higher Cmax, it was not statistically significantly higher (P = 0.068).
Conclusions: The present work demonstrated that administration of capsaicin via the LMP formulation significantly impacted the pharmacokinetic parameters and the serum bioavailability of orally administered 1 mg/kg capsaicin in rats. The bioavailability of capsaicin in humans may also be increased by using the LMP formulation.
© 2021. The Author(s).
Conflict of interest statement
AB, SD, KF, and TW are employees of Lonza, who developed the LMP encapsulations. The other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

