Enhanced slow inactivation contributes to dysfunction of a recurrent SCN2A mutation associated with developmental and epileptic encephalopathy
- PMID: 34287911
- PMCID: PMC8446326
- DOI: 10.1113/JP281834
Enhanced slow inactivation contributes to dysfunction of a recurrent SCN2A mutation associated with developmental and epileptic encephalopathy
Abstract
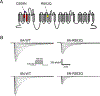
Key points: The recurrent SCN2A mutation R853Q is associated with developmental and epileptic encephalopathy with typical onset after the first months of life. Heterologously expressed R853Q channels exhibit an overall loss-of-function as a result of multiple defects in time- and voltage-dependent channel properties. A previously unrecognized enhancement of slow inactivation is conferred by the R853Q mutation and is a major driver of loss-of-function. Enhanced slow inactivation is potentiated in the canonical splice isoform of the channel and this may explain the later onset of symptoms associated with R853Q.
Abstract: Mutations in voltage gated sodium (NaV ) channel genes, including SCN2A (encoding NaV 1.2), are associated with diverse neurodevelopmental disorders with or without epilepsy that present clinically with varying severity, age-of-onset and pharmacoresponsiveness. We examined the functional properties of the most recurrent SCN2A mutation (R853Q) to determine whether developmentally-regulated alternative splicing impacts dysfunction severity and to investigate effects of the mutation on slow inactivation. We engineered the R853Q mutation into neonatal and adult NaV 1.2 splice isoforms. Channel constructs were heterologously co-expressed in HEK293T cells with human β1 and β2 subunits. Whole-cell patch clamp recording was used to compare time- and voltage-dependent properties of mutant and wild-type channels. The R853Q mutation exhibits an overall loss-of-function attributed to multiple functional defects including a previously undiscovered enhancement of slow inactivation. The mutation exhibited altered voltage dependence of activation and inactivation, slower recovery from inactivation and decreased channel availability during high-frequency depolarizations. More notable were effects on slow inactivation, including a 10-fold slower rate of recovery from slow inactivation exhibited by mutant channels. The impairments in fast inactivation properties were more severe in the neonatal splice isoform, whereas slow inactivation was more pronounced in the splice isoform of the channel expressed predominantly in later childhood. Enhanced later-onset slow inactivation may be a primary driver of the later onset of neurological features associated with this mutation.
Keywords: channelopathy; developmental and epileptic encephalopathy; epilepsy; sodium channel.
© 2021 The Authors. The Journal of Physiology © 2021 The Physiological Society.
Conflict of interest statement
Figures
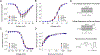


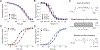

References
-
- Allen AS, Berkovic SF, Cossette P, Delanty N, Dlugos D, Eichler EE, Epstein MP, Glauser T, Goldstein DB, Han Y, Heinzen EL, Hitomi Y, Howell KB, Johnson MR, Kuzniecky R, Lowenstein DH, Lu YF, Madou MR, Marson AG, Mefford HC, Esmaeeli NS, O’Brien TJ, Ottman R, Petrovski S, Poduri A, Ruzzo EK, Scheffer IE, Sherr EH, Yuskaitis CJ, bou-Khalil B, Alldredge BK, Bautista JF, Berkovic SF, Boro A, Cascino GD, Consalvo D, Crumrine P, Devinsky O, Dlugos D, Epstein MP, Fiol M, Fountain NB, French J, Friedman D, Geller EB, Glauser T, Glynn S, Haut SR, Hayward J, Helmers SL, Joshi S, Kanner A, Kirsch HE, Knowlton RC, Kossoff EH, Kuperman R, Kuzniecky R, Lowenstein DH, McGuire SM, Motika PV, Novotny EJ, Ottman R, Paolicchi JM, Parent JM, Park K, Poduri A, Scheffer IE, Shellhaas RA, Sherr EH, Shih JJ, Singh R, Sirven J, Smith MC, Sullivan J, Lin TL, Venkat A, Vining EP, Von Allmen GK, Weisenberg JL, Widdess-Walsh P & Winawer MR. (2013). De novo mutations in epileptic encephalopathies. Nature 501, 217–221. - PMC - PubMed
-
- Bendahhou S, Cummins TR, Kula RW, Fu YH & Ptacek LJ. (2002). Impairment of slow inactivation as a common mechanism for periodic paralysis in DIIS4-S5. Neurology 58, 1266–1272. - PubMed
-
- Berkovic SF, Heron SE, Giordano L, Marini C, Guerrini R, Kaplan RE, Gambardella A, Steinlein OK, Grinton BE, Dean JT, Bordo L, Hodgson BL, Yamamoto T, Mulley JC, Zara F & Scheffer IE. (2004). Benign familial neonatal-infantile seizures: characterization of a new sodium channelopathy. Ann Neurol 55, 550–557. - PubMed
-
- Carvill GL, Heavin SB, Yendle SC, McMahon JM, O’Roak BJ, Cook J, Khan A, Dorschner MO, Weaver M, Calvert S, Malone S, Wallace G, Stanley T, Bye AM, Bleasel A, Howell KB, Kivity S, Mackay MT, Rodriguez-Casero V, Webster R, Korczyn A, Afawi Z, Zelnick N, Lerman-Sagie T, Lev D, Moller RS, Gill D, Andrade DM, Freeman JL, Sadleir LG, Shendure J, Berkovic SF, Scheffer IE & Mefford HC. (2013). Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet 45, 825–830. - PMC - PubMed

