Adding saliva testing to oropharyngeal and deep nasal swab testing increases PCR detection of SARS-CoV-2 in primary care and children
- PMID: 34287935
- PMCID: PMC8447377
- DOI: 10.5694/mja2.51188
Adding saliva testing to oropharyngeal and deep nasal swab testing increases PCR detection of SARS-CoV-2 in primary care and children
Abstract
Objective: To compare the concordance and acceptability of saliva testing with standard-of-care oropharyngeal and bilateral deep nasal swab testing for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in children and in general practice.
Design: Prospective multicentre diagnostic validation study.
Setting: Royal Children's Hospital, and two general practices (cohealth, West Melbourne; Cirqit Health, Altona North) in Melbourne, July-October 2020.
Participants: 1050 people who provided paired saliva and oropharyngeal-nasal swabs for SARS-CoV-2 testing.
Main outcome measures: Numbers of cases in which SARS-CoV-2 was detected in either specimen type by real-time polymerase chain reaction; concordance of results for paired specimens; positive percent agreement (PPA) for virus detection, by specimen type.
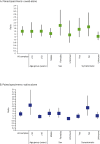
Results: SARS-CoV-2 was detected in 54 of 1050 people with assessable specimens (5%), including 19 cases (35%) in which both specimens were positive. The overall PPA was 72% (95% CI, 58-84%) for saliva and 63% (95% CI, 49-76%) for oropharyngeal-nasal swabs. For the 35 positive specimens from people aged 10 years or more, PPA was 86% (95% CI, 70-95%) for saliva and 63% (95% CI, 45-79%) for oropharyngeal-nasal swabs. Adding saliva testing to standard-of-care oropharyngeal-nasal swab testing increased overall case detection by 59% (95% CI, 29-95%). Providing saliva was preferred to an oropharyngeal-nasal swab by most participants (75%), including 141 of 153 children under 10 years of age (92%).
Conclusion: In children over 10 years of age and adults, saliva testing alone may be suitable for SARS-CoV-2 detection, while for children under 10, saliva testing may be suitable as an adjunct to oropharyngeal-nasal swab testing for increasing case detection.
Keywords: COVID-19; Child health; Diagnosis; General practice; Infectious diseases; Public health; Respiratory tract infections.
© 2021 The Authors. Medical Journal of Australia published by John Wiley & Sons Australia, Ltd on behalf of AMPCo Pty Ltd.
Conflict of interest statement
No relevant disclosures.
Figures
References
-
- Public Health Laboratory Network . PHLN guidance on laboratory testing for SARS‐CoV‐2 (the virus that causes COVID‐19), version 1.16. Updated Feb 2021. https://www.health.gov.au/resources/publications/phln-guidance-on-labora... (viewed May 2021).
-
- Fernandes LL, Pacheco VB, Borges L, et al. Saliva in the diagnosis of COVID‐19: a review and new research directions. J Dent Res 2020; 99: 1435–1443. - PubMed
-
- Kivelä JM, Jarva H, Lappalainen M, Kurkela S. Saliva‐based testing for diagnosis of SARS‐CoV‐2 infection: a meta‐analysis. J Med Virol 2021; 93: 1256–1258. - PubMed
-
- Buban JM, Villanueva PN, Gregorio GEV. Should RT‐PCR of saliva samples be used for diagnosis of COVID‐19? (Philippine COVID‐19 Living Clinical Practice Guidelines). Updated 15 Mar 2021. https://www.psmid.org/wp-content/uploads/2021/05/SALIVA-RT-PCR-CPG-FINAL... (viewed May 2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous