Application of an integrated radio-frequency/shim coil technology for signal recovery in fMRI
- PMID: 34288086
- PMCID: PMC8568668
- DOI: 10.1002/mrm.28925
Application of an integrated radio-frequency/shim coil technology for signal recovery in fMRI
Abstract
Purpose: Gradient-echo echo-planar imaging (EPI), which is typically used for blood oxygenation level-dependent (BOLD) functional MRI (fMRI), suffers from distortions and signal loss caused by localized B0 inhomogeneities. Such artifacts cannot be effectively corrected for with the low-order spherical harmonic (SH) shim coils available on most scanners. The integrated parallel reception, excitation, and shimming (iPRES) coil technology allows radiofrequency (RF) and direct currents to flow on each coil element, enabling imaging and localized B0 shimming with one coil array. iPRES was previously used to correct for distortions in spin-echo EPI and is further developed here to also recover signal loss in gradient-echo EPI.
Methods: The cost function in the shim optimization, which typically uses a single term representing the B0 inhomogeneity, was modified to include a second term representing the signal loss, with an adjustable weight to optimize the trade-off between distortion correction and signal recovery. Simulations and experiments were performed to investigate the shimming performance.
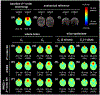
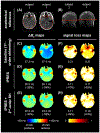
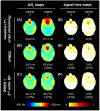
Results: Slice-optimized shimming with iPRES and the proposed cost function substantially reduced the signal loss in the inferior frontal and temporal brain regions compared to shimming with iPRES and the original cost function or 2nd -order SH shimming with either cost function. In breath-holding fMRI experiments, the ΔB0 and signal loss root-mean-square errors decreased by -34.3% and -56.2%, whereas the EPI signal intensity and number of activated voxels increased by 60.3% and 174.0% in the inferior frontal brain region.
Conclusion: iPRES can recover signal loss in gradient-echo EPI, which is expected to improve BOLD fMRI studies in brain regions suffering from signal loss.
Keywords: B0 shimming; BOLD fMRI; gradient-echo EPI; human brain; iPRES; signal recovery.
© 2021 International Society for Magnetic Resonance in Medicine.
Figures





References
-
- Constable RT. Functional MR imaging using gradient-echo echo-planar imaging in the presence of large static field inhomogeneities. J Magn Reson Imaging 1995;5:746–752. - PubMed
-
- Glover GH. 3D z-shim method for reduction of susceptibility effects in BOLD fMRI. Magn Reson Med. 1999;42(2):290–299. - PubMed
-
- Song AW. Single-shot EPI with signal recovery from the susceptibility-induced losses. Magn Reson Med. 2001;46(2):407–411. - PubMed
-
- Truong TK, Song AW. Single-shot dual-z-shimmed sensitivity-encoded spiral-in/out imaging for functional MRI with reduced susceptibility artifacts. Magn Reson Med. 2008;59(1):221–7. - PubMed
-
- Hsu JJ, Glover GH. Mitigation of susceptibility-induced signal loss in neuroimaging using localized shim coils. Magn Reson Med. 2005;53(2):243–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

