Transplantation programs facing lack of empirical evidence on SARS-CoV-2 vaccination: A society recommendation consensus update
- PMID: 34288294
- PMCID: PMC8420432
- DOI: 10.1111/tid.13696
Transplantation programs facing lack of empirical evidence on SARS-CoV-2 vaccination: A society recommendation consensus update
Abstract
Background: Since phase III trials for the most prominent vaccines excluded immunocompromised or immunosuppressed patients, data on safety and efficacy of SARS-CoV-2 vaccines for recipients of solid organ transplantations are scarce.
Aims: Our study offers a synthesis of expert opinions aligned with available data addressing key questions of the clinical management of SARS-CoV-2 vaccinations for transplant patients.
Method: An online research was performed retrieving available recommendations by national and international transplantation organizations and state institutions on SARS-CoV2 vaccination management for transplant recipients.
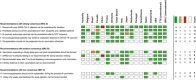
Results: Eleven key statements were identified from recommendations by 18 national and international societies, and consensus for the individual statements was evaluated by means of the Society Recommendation Consensus score. The highest consensus level (SRC A) was found for prioritized access to vaccination for transplant patients despite anticipation of a weakened immune response. All currently authorized vaccines can be considered safe for transplant patients (SRC A). The handling of immunosuppressive medication, the timely management of vaccines, and other aspects were aligned with available expert opinions.
Conclusion: Expert consensus can be determined for crucial aspects of the implementation of SARS-CoV-2 vaccination programs. We hereby offer a tool for immediate decision-making until empirical data becomes available.
Keywords: COVID-19; SARS-CoV-2 vaccination; solid organ transplantation.
© 2021 The Authors. Transplant Infectious Disease published by Wiley Periodicals LLC.
Conflict of interest statement
Moritz Schmelzle received funding from Merck Serono GmbH, Bayer AG, Amgen Inc., ERBE Elektromedizin GmbH, Johnson&Johnson Medical GmbH, Takeda Pharm. Lim., Olympus K.K., Medtronic GmbH, and Intuitive Surg. Inc. All other authors declare no conflict of interest.
Figures
References
-
- COVID‐19 vaccine FAQ sheet. American Society of Transplantation . Accessed April 18, 2021. https://www.myast.org/sites/default/files/2021%2003%2018%20COVID19%20VAC...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

