COVID-19: Thrombosis, thromboinflammation, and anticoagulation considerations
- PMID: 34288441
- PMCID: PMC8444926
- DOI: 10.1111/ijlh.13500
COVID-19: Thrombosis, thromboinflammation, and anticoagulation considerations
Abstract
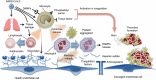
Vascular endothelial injury is a hallmark of acute infection at both the microvascular and macrovascular levels. The hallmark of SARS-CoV-2 infection is the current COVID-19 clinical sequelae of the pathophysiologic responses of hypercoagulability and thromboinflammation associated with acute infection. The acute lung injury that initially occurs in COVID-19 results from vascular and endothelial damage from viral injury and pathophysiologic responses that produce the COVID-19-associated coagulopathy. Clinicians should continue to focus on the vascular endothelial injury that occurs and evaluate potential therapeutic interventions that may benefit those with new infections during the current pandemic as they may also be of benefit for future pathogens that generate similar thromboinflammatory responses. The current Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) studies are important projects that will further define our management strategies. At the time of writing this report, two mRNA vaccines are now being distributed and will hopefully have a major impact on slowing the global spread and subsequent thromboinflammatory injury we see clinically in critically ill patients.
Keywords: COVID-19; anticoagulant therapy; coagulopathy; disseminated intravascular coagulation; endothelial cell; thrombosis.
© 2021 John Wiley & Sons Ltd.
Conflict of interest statement
JHL serves on research, data safety, or advisory committees for Instrumentation Labs, Merck, and Octapharma. TI provided research grants from Japan Blood Products Organization and JIMRO. LO and KMC have no COIs. JMC received personal fees from Bristol‐Myer Squibb, Abbott, Portola, and Pfizer. This study provided research funding to the institution from CSL Behring.
Figures

References
-
- Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133(9):906‐918. - PubMed
-
- Iba T, Levy JH, Wada H, et al. Differential diagnoses for sepsis‐induced disseminated intravascular coagulation: communication from the SSC of the ISTH. J Thromb Haemost. 2019;17(2):415‐419. - PubMed
-
- Iba T, Levy JH, Warkentin TE, et al. Diagnosis and management of sepsis‐induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989‐1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

