Siglec-6 is a novel target for CAR T-cell therapy in acute myeloid leukemia
- PMID: 34289026
- PMCID: PMC9642786
- DOI: 10.1182/blood.2020009192
Siglec-6 is a novel target for CAR T-cell therapy in acute myeloid leukemia
Abstract
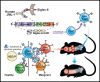
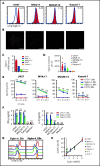
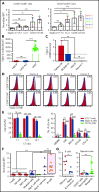
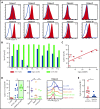
Acute myeloid leukemia (AML) is an attractive entity for the development of chimeric antigen receptor (CAR) T-cell immunotherapy because AML blasts are susceptible to T-cell-mediated elimination. Here, we introduce sialic acid-binding immunoglobulin-like lectin 6 (Siglec-6) as a novel target for CAR T cells in AML. We designed a Siglec-6-specific CAR with a targeting domain derived from the human monoclonal antibody JML-1. We found that Siglec-6 is commonly expressed on AML cell lines and primary AML blasts, including the subpopulation of AML stem cells. Treatment with Siglec-6 CAR T cells confers specific antileukemia reactivity that correlates with Siglec-6 expression in preclinical models, including induction of complete remission in a xenograft AML model in immunodeficient mice (NSG/U937). In addition, we confirmed Siglec-6 expression on transformed B cells in chronic lymphocytic leukemia (CLL), and specific anti-CLL reactivity of Siglec-6 CAR T cells in vitro. Of particular interest, we found that Siglec-6 is not detectable on normal hematopoietic stem and progenitor cells (HSPCs) and that treatment with Siglec-6 CAR T cells does not affect their viability and lineage differentiation in colony-formation assays. These data suggest that Siglec-6 CAR T-cell therapy may be used to effectively treat AML without the need for subsequent allogeneic hematopoietic stem cell transplantation. In mature normal hematopoietic cells, we detected Siglec-6 in a proportion of memory (and naïve) B cells and basophilic granulocytes, suggesting the potential for limited on-target/off-tumor reactivity. The lack of expression of Siglec-6 on normal HSPCs is a key to differentiating it from other Siglec family members (eg, Siglec-3 [CD33]) and other CAR target antigens (eg, CD123) that are under investigation in AML, and it warrants the clinical investigation of Siglec-6 CAR T-cell therapy.
© 2021 by The American Society of Hematology.
Figures


Comment in
-
Siglec-6 CAR T: magic bullet for a moving target.Blood. 2021 Nov 11;138(19):1786-1787. doi: 10.1182/blood.2021013184. Blood. 2021. PMID: 34762132 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

