Comparison between physiologically based pharmacokinetic and population pharmacokinetic modelling to select paediatric doses of gepotidacin in plague
- PMID: 34289143
- PMCID: PMC9293063
- DOI: 10.1111/bcp.14996
Comparison between physiologically based pharmacokinetic and population pharmacokinetic modelling to select paediatric doses of gepotidacin in plague
Abstract
Aims: To develop physiologically based pharmacokinetic (PBPK) and population pharmacokinetic (PopPK) models to predict effective doses of gepotidacin in paediatrics for the treatment of pneumonic plague (Yersinia pestis).
Methods: A gepotidacin PBPK model was constructed using a population-based absorption, distribution, metabolism and excretion simulator, Simcyp®, with physicochemical and in vitro data, optimized with clinical data from a dose-escalation intravenous (IV) study and a human mass balance study. A PopPK model was developed with pooled PK data from phase 1 studies with IV gepotidacin in healthy adults.
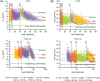
Results: For both the PopPK and PBPK models, body weight was found to be a key covariate affecting gepotidacin clearance. With PBPK, ~90% of the predicted PK for paediatrics fell between the 5th and 95th percentiles of adult values except for subjects weighing ≤5 kg. PopPK-simulated paediatric means for Cmax and AUC(0-τ) were similar to adult exposures across various weight brackets. The proposed dosing regimens were weight-based for subjects ≤40 kg and fixed-dose for subjects >40 kg. Comparison of observed and predicted exposures in adults indicated that both PBPK and PopPK models achieved similar AUC and Cmax for a given dose, but the Cmax predictions with PopPK were slightly higher than with PBPK. The two models differed on dose predictions in children <3 months old. The PopPK model may be suboptimal for low age groups due to the absence of maturation characterization of drug-metabolizing enzymes involved with clearance in adults.
Conclusions: Both PBPK and PopPK approaches can reasonably predict gepotidacin exposures in children.
Keywords: PBPK; modelling; pharmacodynamics; population analysis; simulation.
© 2021 Glaxo Group Limited. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
This study was funded by GSK. All authors were employees of GSK when this work was completed and meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. C.A. is currently at Gan & Lee Pharmaceuticals, Bridgewater, New Jersey, USA. M.H. is currently at Agios Pharmaceuticals, Cambridge, Massachusetts, USA. D.G. is currently at CSL Behring, 1020 1st Ave, King of Prussia, PA 19406. There was no principal investigator for this study since this work presents an analysis of data collected from previous clinical trials. All of the previous clinical trials were conducted according to the ethical principles of “Good Clinical Practice” and the Declaration of Helsinki after obtaining written informed consent from each subject. In addition, all pertinent protocols were approved by an accredited investigational review board or ethics committee.
Data sharing is not applicable for this publication; no new clinical data were generated in this modelling study.
Figures


References
-
- World Health Organization . Antibiotic Resistance. November 2017. http://www.who.int/mediacentre/factsheets/antibiotic-resistance/en/ Accessed 7 March 2018.
-
- Biedenbach DJ, Bouchillon SK, Hackel M, et al. In vitro activity of gepotidacin, a novel triazaacenaphthylene bacterial topoisomerase inhibitor, against a broad spectrum of bacterial pathogens. Antimicrob Agents Chemother. 2016;60(3):1918‐1923. 10.1128/AAC.02820-15 PMID: 26729499; PMCID: PMC4776004 - DOI - PMC - PubMed
-
- Scangarella‐Oman N, Hossain M, Dixon P, et al. P2.38 Microbiological analysis from a phase II study in adults evaluating single doses of gepotidacin (GSK2140944) in the treatment of uncomplicated urogenital gonorrhoea caused by Neisseria gonorrhoeae . Sex Transm Infect. 2017;93:A84‐A85. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

