Bacterial lysate add-on therapy to reduce exacerbations in severe asthma: A double-blind placebo-controlled trial
- PMID: 34289183
- PMCID: PMC9292626
- DOI: 10.1111/cea.13990
Bacterial lysate add-on therapy to reduce exacerbations in severe asthma: A double-blind placebo-controlled trial
Abstract
Background: Asthma exacerbations are frequently induced by respiratory tract infections (RTIs). Bacterial lysates have been described to possess immune-modulatory effects and reduce RTIs as well as asthma symptoms in children. However, whether bacterial lysates have similar effects in adult asthma patients is unknown.
Aims: To reduce asthma exacerbations by add-on bacterial lysate therapy in adults with severe asthma and to characterize the clinical and immune-modulatory effects of this treatment.
Methods: Asthma patients (GINA 4) with ≥2 annual exacerbations in the previous year were included. The intervention regimen consisted of OM-85/placebo for 10 consecutive days per month for 6 months during two winter seasons. Primary end-point was the number of severe asthma exacerbations within 18 months. The study was approved by the national and local ethical review board and registered in the Dutch Trial Registry (NL5752). All participants provided written informed consent.
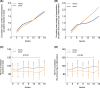
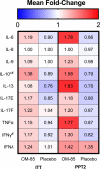
Results: Seventy-five participants were included (38 OM-85; 37 placebo). Exacerbation frequencies were not different between the groups after 18 months (incidence rate ratio 1.07, 95%CI [0.68-1.69], p = 0.77). With the use of OM-85, FEV1% increased by 3.81% (p = 0.04) compared with placebo. Nasopharyngeal swabs taken during RTIs detected a virus less frequently in patients using OM-85 compared to placebo (30.5% vs. 48.0%, p = 0.02). In subjects with type 2 inflammation adherent to the protocol (22 OM-85; 20 placebo), a non-statistically significant decrease in exacerbations in the OM-85 group was observed (IRR = 0.71, 95%CI [0.39-1.26], p = 0.25). Immune-modulatory effects included an increase in several plasma cytokines in the OM-85 group, especially IL-10 and interferons. Peripheral blood T- and B cell subtyping, including regulatory T cells, did not show differences between the groups.
Conclusion: Although OM-85 may have immune-modulatory effects, it did not reduce asthma exacerbations in this heterogeneous severe adult asthma group. Post hoc analysis showed a potential clinical benefit in patients with type 2 inflammation.
Keywords: asthma; bacterial lysates; exacerbations; immune modulation; type 2 inflammation.
© 2021 The Authors. Clinical & Experimental Allergy published by John Wiley & Sons Ltd.
Conflict of interest statement
GT reports speaker fees and investigator‐initiated grant support from OM Pharma and Astra Zeneca, not paid in person but directly to a research foundation. GJB has received grant/research support for consultations and/or speaking at conferences from Novartis, GSK, AstraZeneca, ALK, Teva, Sanofi and Chiesi. GMB, EKP, CMZ, AB, GE, MN, JWB, GV, EB, BMBK, RS and RWH have no conflicts of interest.
Figures

References
-
- Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783‐800. - PubMed
-
- Esposito S, Soto‐Martinez ME, Feleszko W, Jones MH, Shen KL, Schaad UB. Nonspecific immunomodulators for recurrent respiratory tract infections, wheezing and asthma in children: a systematic review of mechanistic and clinical evidence. Curr Opin Allergy Clin Immunol. 2018;18(3):198‐209. - PMC - PubMed

