MRI findings in-between leiomyoma and leiomyosarcoma: a Rad-Path correlation of degenerated leiomyomas and variants
- PMID: 34289327
- PMCID: PMC9327769
- DOI: 10.1259/bjr.20210283
MRI findings in-between leiomyoma and leiomyosarcoma: a Rad-Path correlation of degenerated leiomyomas and variants
Abstract
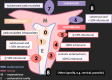
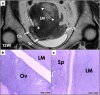
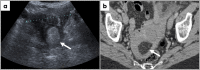
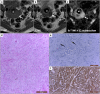
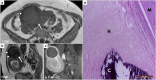
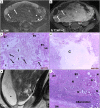
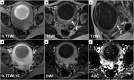
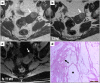
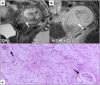
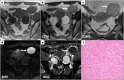
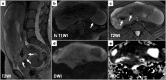
Leiomyomas are the most common benign tumors of the uterus. On the opposite side, leiomyosarcomas are rare malignant uterine tumors that account for a significant proportion of uterine cancer deaths. Especially when large and degenerated, leiomyomas and leiomyoma variants can have overlapping imaging characteristics with those of leiomyosarcomas. Although not always possible, it is paramount to be able to differentiate between leiomyomas and leiomyosarcomas on imaging, as the therapeutic management can differ. This pictorial review aims to familiarize radiologists with imaging features of leiomyomas and various types of leiomyoma degeneration and variants, together with their pathology correlates.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

