Tau oligomer induced HMGB1 release contributes to cellular senescence and neuropathology linked to Alzheimer's disease and frontotemporal dementia
- PMID: 34289368
- PMCID: PMC8341760
- DOI: 10.1016/j.celrep.2021.109419
Tau oligomer induced HMGB1 release contributes to cellular senescence and neuropathology linked to Alzheimer's disease and frontotemporal dementia
Abstract
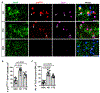
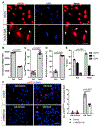
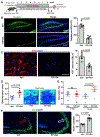
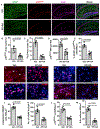
Aging, pathological tau oligomers (TauO), and chronic inflammation in the brain play a central role in tauopathies, including Alzheimer's disease (AD) and frontotemporal dementia (FTD). However, the underlying mechanism of TauO-induced aging-related neuroinflammation remains unclear. Here, we show that TauO-associated astrocytes display a senescence-like phenotype in the brains of patients with AD and FTD. TauO exposure triggers astrocyte senescence through high mobility group box 1 (HMGB1) release and inflammatory senescence-associated secretory phenotype (SASP), which mediates paracrine senescence in adjacent cells. HMGB1 release inhibition using ethyl pyruvate (EP) and glycyrrhizic acid (GA) prevents TauO-induced senescence through inhibition of p38-mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF-κB)-the essential signaling pathways for SASP development. Despite the developed tauopathy in 12-month-old hTau mice, EP+GA treatment significantly decreases TauO and senescent cell loads in the brain, reduces neuroinflammation, and thus ameliorates cognitive functions. Collectively, TauO-induced HMGB1 release promotes cellular senescence and neuropathology, which could represent an important common pathomechanism in tauopathies including AD and FTD.
Keywords: HMGB1; SASP; aging; astrocytes; cognitive functions; neurodegeneration; neuroinflammation; senescence; tau oligomers; tauopathies.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, and Davies P (2003). Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem 86, 582–590. - PubMed
-
- Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, and Blennow K (2001). Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch. Neurol 58, 373–379. - PubMed
-
- Arélin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, and Hyman BT (2002). LRP and senile plaques in Alzheimer’s disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res. Mol. Brain Res 104, 38–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

