Rodent genetic models of Ah receptor signaling
- PMID: 34289754
- PMCID: PMC9059429
- DOI: 10.1080/03602532.2021.1955916
Rodent genetic models of Ah receptor signaling
Abstract
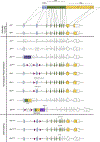
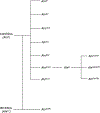
The aryl hydrocarbon receptor (AHR) is a ligand activated transcription factor that is a member of the PER-ARNT-SIM superfamily of environmental sensors. This receptor has been a molecule of interest for many years in the field of toxicology, as it was originally discovered to mediate the toxic effects of certain environmental pollutants like benzo(a)pyrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin. While all animals express this protein, there is naturally occurring variability in receptor size and responsiveness to ligand. This naturally occurring variation, particularly in mice, has been an essential tool in the discovery and early characterization of the AHR. Genetic models including congenic mice and induced mutations at the Ahr locus have proven invaluable in further understanding the role of the AHR in adaptive metabolism and TCDD-induced toxicity. The creation and examination of Ahr null mice revealed an important physiological role for the AHR in vascular and hepatic development and mediation of the immune system. In this review, we attempt to provide an overview to many of the AHR models that have aided in the understanding of AHR biology thus far. We describe the naturally occurring polymorphisms, congenic models, induced mutations at the Ahr locus and at the binding partner Ah Receptor Nuclear Translocator and chaperone, Ah receptor associated 9 loci in mice, with a brief description of naturally occurring and induced mutations in rats.
Keywords: AHR; ARA9; ARNT; aryl hydrocarbon receptor; gene editing; mouse model; rat model.
Figures



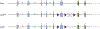
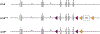
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
