Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART)
- PMID: 34289996
- PMCID: PMC8293749
- DOI: 10.1136/bmj.n1647
Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART)
Erratum in
-
Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART).BMJ. 2021 Aug 26;374:n2116. doi: 10.1136/bmj.n2116. BMJ. 2021. PMID: 34446438 Free PMC article. No abstract available.
Abstract
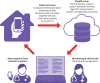
Objective: To evaluate effects of remote monitoring of adjuvant chemotherapy related side effects via the Advanced Symptom Management System (ASyMS) on symptom burden, quality of life, supportive care needs, anxiety, self-efficacy, and work limitations.
Design: Multicentre, repeated measures, parallel group, evaluator masked, stratified randomised controlled trial.
Setting: Twelve cancer centres in Austria, Greece, Norway, Republic of Ireland, and UK.
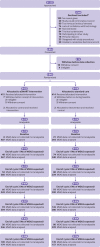
Participants: 829 patients with non-metastatic breast cancer, colorectal cancer, Hodgkin's disease, or non-Hodgkin's lymphoma receiving first line adjuvant chemotherapy or chemotherapy for the first time in five years.
Intervention: Patients were randomised to ASyMS (intervention; n=415) or standard care (control; n=414) over six cycles of chemotherapy.
Main outcome measures: The primary outcome was symptom burden (Memorial Symptom Assessment Scale; MSAS). Secondary outcomes were health related quality of life (Functional Assessment of Cancer Therapy-General; FACT-G), Supportive Care Needs Survey Short-Form (SCNS-SF34), State-Trait Anxiety Inventory-Revised (STAI-R), Communication and Attitudinal Self-Efficacy scale for cancer (CASE-Cancer), and work limitations questionnaire (WLQ).
Results: For the intervention group, symptom burden remained at pre-chemotherapy treatment levels, whereas controls reported an increase from cycle 1 onwards (least squares absolute mean difference -0.15, 95% confidence interval -0.19 to -0.12; P<0.001; Cohen's D effect size=0.5). Analysis of MSAS sub-domains indicated significant reductions in favour of ASyMS for global distress index (-0.21, -0.27 to -0.16; P<0.001), psychological symptoms (-0.16, -0.23 to -0.10; P<0.001), and physical symptoms (-0.21, -0.26 to -0.17; P<0.001). FACT-G scores were higher in the intervention group across all cycles (mean difference 4.06, 95% confidence interval 2.65 to 5.46; P<0.001), whereas mean scores for STAI-R trait (-1.15, -1.90 to -0.41; P=0.003) and STAI-R state anxiety (-1.13, -2.06 to -0.20; P=0.02) were lower. CASE-Cancer scores were higher in the intervention group (mean difference 0.81, 0.19 to 1.43; P=0.01), and most SCNS-SF34 domains were lower, including sexuality needs (-1.56, -3.11 to -0.01; P<0.05), patient care and support needs (-1.74, -3.31 to -0.16; P=0.03), and physical and daily living needs (-2.8, -5.0 to -0.6; P=0.01). Other SCNS-SF34 domains and WLQ were not significantly different. Safety of ASyMS was satisfactory. Neutropenic events were higher in the intervention group.
Conclusions: Significant reduction in symptom burden supports the use of ASyMS for remote symptom monitoring in cancer care. A "medium" Cohen's effect size of 0.5 showed a sizable, positive clinical effect of ASyMS on patients' symptom experiences. Remote monitoring systems will be vital for future services, particularly with blended models of care delivery arising from the covid-19 pandemic.
Trial registration: Clinicaltrials.gov NCT02356081.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: financial support from the European Commission for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
