A human CD137×PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade
- PMID: 34290245
- PMCID: PMC8295259
- DOI: 10.1038/s41467-021-24767-5
A human CD137×PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade
Abstract
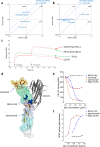
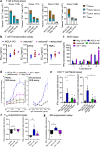
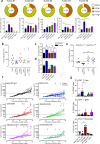
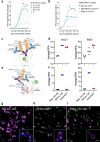
Immune checkpoint inhibitors demonstrate clinical activity in many tumor types, however, only a fraction of patients benefit. Combining CD137 agonists with these inhibitors increases anti-tumor activity preclinically, but attempts to translate these observations to the clinic have been hampered by systemic toxicity. Here we describe a human CD137xPD-L1 bispecific antibody, MCLA-145, identified through functional screening of agonist- and immune checkpoint inhibitor arm combinations. MCLA-145 potently activates T cells at sub-nanomolar concentrations, even under suppressive conditions, and enhances T cell priming, differentiation and memory recall responses. In vivo, MCLA-145 anti-tumor activity is superior to immune checkpoint inhibitor comparators and linked to recruitment and intra-tumor expansion of CD8 + T cells. No graft-versus-host-disease is observed in contrast to other antibodies inhibiting the PD-1 and PD-L1 pathway. Non-human primates treated with 100 mg/kg/week of MCLA-145 show no adverse effects. The conditional activation of CD137 signaling by MCLA-145, triggered by neighboring cells expressing >5000 copies of PD-L1, may provide both safety and potency advantages.
© 2021. The Author(s).
Conflict of interest statement
C.G., P.T., R.K., P.L., A.Kr, L.J.H., H.M., E.R., S.E., F.F., R.B., V.Z.vZ., A.B., W.B., W.M., A.B.H.B., T.L., J.K., and M.T. are employees of Merus N.V. L.C.W., J.Z., A.M., C.H., T.C., A.V., C.K., A.Ku, Y.L., L.H., S.H., S.S., H.N., P.S., G.H., R.H., and P.M. are employees of Incyte Inc. C.G., M.T., P.T., R.K., J.K., and T.L. are inventors on intellectual property related to this work. E.M. and S.A. received grant funding from Incyte. The remaining authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

