Selective activation of pro-anti-IL-1β antibody enhances specificity for autoinflammatory disorder therapy
- PMID: 34290297
- PMCID: PMC8295355
- DOI: 10.1038/s41598-021-94298-y
Selective activation of pro-anti-IL-1β antibody enhances specificity for autoinflammatory disorder therapy
Abstract
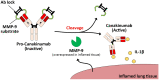
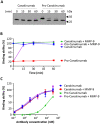
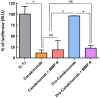
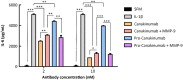
Canakinumab is a fully human monoclonal antibody that specifically neutralizes human interleukin (IL)-1β and has been approved by the US Food and Drug Administration for treating different types of autoinflammatory disorders such as cryopyrin-associated periodic syndrome, tumor necrosis factor receptor-associated periodic syndrome and systemic juvenile idiopathic arthritis. However, long-term systemic neutralization of IL-1β by Canakinumab may cause severe adverse events such as serious upper respiratory tract infections and inflammation, thereby decreasing the quality of life of patients. Here, we used an IgG1 hinge as an Ab lock to cover the IL-1β-binding site of Canakinumab by linking with matrix metalloprotease 9 (MMP-9) substrate to generate pro-Canakinumab that can be specifically activated in the inflamed regions in autoinflammatory diseases to enhance the selectivity and safety of treatment. The Ab lock significantly inhibited the IL-1β-binding by 68-fold compared with Canakinumab, and MMP-9 completely restored the IL-1β neutralizing ability of pro-Canakinumab within 60 min and blocked IL-1β-downstream signaling and IL-1β-regulated genes (i.e., IL-6). It is expected that MMP-9 cleavable and efficient Ab lock will be able to significantly enhance the selective reaction of Canakinumab at the disease site and reduce the on-target toxicities of Canakinumab during systemic circulation, thereby showing potential for development to improve the safety and quality of life of patients with autoinflammatory disorders in the future.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- De Benedetti F, Gattorno M, Anton J, Ben-Chetrit E, Frenkel J, Hoffman HM, Kone-Paut I, Lachmann HJ, Ozen S, Simon A, Zeft A, Calvo Penades I, Moutschen M, Quartier P, Kasapcopur O, Shcherbina A, Hofer M, Hashkes PJ, Van der Hilst J, Hara R, Bujan-Rivas S, Constantin T, Gul A, Livneh A, Brogan P, Cattalini M, Obici L, Lheritier K, Speziale A, Junge G. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med. 2018;378(20):1908–1919. doi: 10.1056/NEJMoa1706314. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

