Structure-function analysis of purified proanthocyanidins reveals a role for polymer size in suppressing inflammatory responses
- PMID: 34290357
- PMCID: PMC8295316
- DOI: 10.1038/s42003-021-02408-3
Structure-function analysis of purified proanthocyanidins reveals a role for polymer size in suppressing inflammatory responses
Abstract
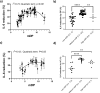
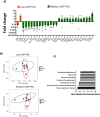
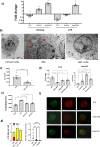
Proanthocyanidins (PAC) are dietary compounds that have been extensively studied for beneficial health effects due to their anti-inflammatory properties. However, the structure-function relationships of PAC and their mode-of-action remain obscure. Here, we isolated a wide range of diverse PAC polymer mixtures of high purity from plant material. Polymer size was a key factor in determining the ability of PAC to regulate inflammatory cytokine responses in murine macrophages. PAC polymers with a medium (9.1) mean degree of polymerization (mDP) induced substantial transcriptomic changes, whereas PAC with either low (2.6) or high (12.3) mDP were significantly less active. Short-term oral treatment of mice with PAC modulated gene pathways connected to nutrient metabolism and inflammation in ileal tissue in a polymerization-dependent manner. Mechanistically, the bioactive PAC polymers modulated autophagic flux and inhibited lipopolysaccharide-induced autophagy in macrophages. Collectively, our results highlight the importance of defined structural features in the health-promoting effects of PAC-rich foods.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Mathers, J. & Wolever, T. M. Introduction to Human Nutrition (Wiley-Blackwell, 2009).
-
- Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

