Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom
- PMID: 34290390
- PMCID: PMC8294260
- DOI: 10.1038/s41564-021-00947-3
Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom
Abstract
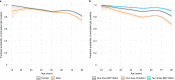
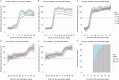
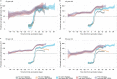
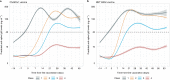
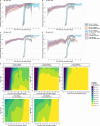
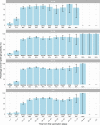
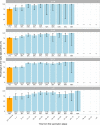
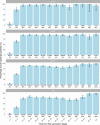
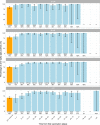
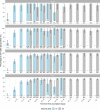
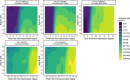
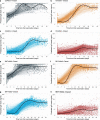
We report that in a cohort of 45,965 adults, who were receiving either the ChAdOx1 or the BNT162b2 SARS-CoV-2 vaccines, in those who had no prior infection with SARS-CoV-2, seroconversion rates and quantitative antibody levels after a single dose were lower in older individuals, especially in those aged >60 years. Two vaccine doses achieved high responses across all ages. Antibody levels increased more slowly and to lower levels with a single dose of ChAdOx1 compared with a single dose of BNT162b2, but waned following a single dose of BNT162b2 in older individuals. In descriptive latent class models, we identified four responder subgroups, including a 'low responder' group that more commonly consisted of people aged >75 years, males and individuals with long-term health conditions. Given our findings, we propose that available vaccines should be prioritized for those not previously infected and that second doses should be prioritized for individuals aged >60 years. Further data are needed to better understand the extent to which quantitative antibody responses are associated with vaccine-mediated protection.
© 2021. The Author(s).
Conflict of interest statement
D.W.E. declares lecture fees from Gilead, outside the submitted work. The remaining authors declare no competing interests.
Figures



References
-
- Medicines and Healthcare products Regulatory Agency. Regulatory Approval of Pfizer/BioNTech Vaccine for COVID-19 (GOV.UK, 2020); https://www.gov.uk/government/publications/regulatory-approval-of-pfizer...
-
- Medicines and Healthcare products Regulatory Agency. Oxford University/AstraZeneca COVID-19 Vaccine Approved (GOV.UK, 2020); https://www.gov.uk/government/news/oxford-universityastrazeneca-covid-19...
-
- UK COVID-19 Vaccines Delivery Plan Contents (Department of Health and Social Care, 2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

