Glycan Nanostructures of Human Coronaviruses
- PMID: 34290504
- PMCID: PMC8289332
- DOI: 10.2147/IJN.S302516
Glycan Nanostructures of Human Coronaviruses
Abstract
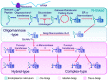
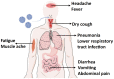
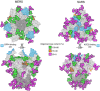
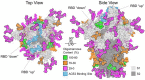
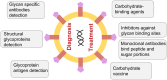
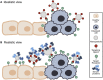
Human coronaviruses present a substantial global disease burden, causing damage to populations' health, economy, and social well-being. Glycans are one of the main structural components of all microbes and organismic structures, including viruses-playing multiple essential roles in virus infection and immunity. Studying and understanding virus glycans at the nanoscale provide new insights into the diagnosis and treatment of viruses. Glycan nanostructures are considered potential targets for molecular diagnosis, antiviral therapeutics, and the development of vaccines. This review article describes glycan nanostructures (eg, glycoproteins and glycolipids) that exist in cells, subcellular structures, and microbes. We detail the structure, characterization, synthesis, and functions of virus glycans. Furthermore, we describe the glycan nanostructures of different human coronaviruses, such as human coronavirus 229E (HCoV-229E), human coronavirus OC43 (HCoV-OC43), severe acute respiratory syndrome-associated coronavirus (SARS-CoV), human coronavirus NL63 (HCoV-NL63), human coronavirus HKU1 (HCoV-HKU1), the Middle East respiratory syndrome-associated coronavirus (MERS-CoV), and how glycan nanotechnology can be useful to prevent and combat human coronaviruses infections, along with possibilities that are not yet explored.
Keywords: SARS-CoV-2; coronaviruses; diagnostics; glycan; glycolipids; glycoproteins; nanotechnology; vaccine.
© 2021 Guo et al.
Conflict of interest statement
The authors declare no conflicts of interest for this work.
Figures





References
-
- Takahashi T, Suzuki T. Role of Glycans in Viral Infection, in Sugar Chains. Springer; 2015:71–93.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

