Assessing the Functional Heterogeneity of Monocytes in Human Septic Shock: a Proof-of-Concept Microfluidic Assay of TNFα Secretion
- PMID: 34290706
- PMCID: PMC8288100
- DOI: 10.3389/fimmu.2021.686111
Assessing the Functional Heterogeneity of Monocytes in Human Septic Shock: a Proof-of-Concept Microfluidic Assay of TNFα Secretion
Abstract
Objective: The development of advanced single-cell technologies to decipher inter-cellular heterogeneity has enabled the dynamic assessment of individual cells behavior over time, overcoming the limitation of traditional assays. Here, we evaluated the feasibility of an advanced microfluidic assay combined to fluorescence microscopy to address the behavior of circulating monocytes from septic shock patients.
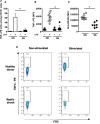
Methods: Seven septic shock patients and ten healthy volunteers were enrolled in the study. Using the proposed microfluidic assay we investigated the production over time of LPS-elicited TNFα by single monocytes encapsulated within droplets. Cellular endocytic activity was assessed by internalization of magnetic nanoparticles. Besides, we assessed HLA-DR membrane expression and LPS-induced TNFα production in monocytes through classical flow cytometry assays.
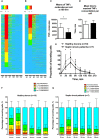
Results: Consistent with the flow cytometry results, the total number of TNFα molecules secreted by encapsulated single monocytes was significantly decreased in septic shock patients compared to healthy donors. TNFα production was dampened as soon as 30 and 60 minutes after LPS stimulation in monocytes from septic patients. Furthermore, the microfluidic assay revealed heterogeneous individual behavior of monocytes from septic shock patients. Of note, monocytes from both healthy donors and patients exhibited similar phagocytic activities over time.
Conclusion: The microfluidic assay highlights the functional heterogeneity of monocytes, and provides in-depth resolution in assessing the hallmark monocyte deactivation encountered in post-septic immunosuppression.
Keywords: immune suppression; microfluidic; monocyte; septic shock; tolerance.
Copyright © 2021 Llitjos, Bounab, Rousseau, Dixneuf, Rimbault, Chiche, Textoris, Pène and Védrine.
Conflict of interest statement
JT is an employee of bioMérieux, S.A. YB and CV are inventors on a patent application based on certain ideas described in this manuscript and may receive financial compensation via their employer’s rewards to inventors’ scheme. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

