microRNAs involved in psoriasis and cardiovascular diseases
- PMID: 34291190
- PMCID: PMC8284950
- DOI: 10.1530/VB-21-0007
microRNAs involved in psoriasis and cardiovascular diseases
Abstract
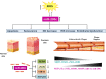
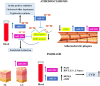
Psoriasis is a chronic inflammatory disease involving the skin. Both genetic and environmental factors play a pathogenic role in psoriasis and contribute to the severity of the disease. Psoriasis, in fact, has been associated with different comorbidities such as diabetes, metabolic syndrome, gastrointestinal or kidney diseases, cardiovascular disease (CVD), and cerebrovascular diseases (CeVD). Indeed, life expectancy in severe psoriasis is reduced by up to 5 years due to CVD and CeVD. Moreover, patients with severe psoriasis have a higher prevalence of traditional cardiovascular (CV) risk factors, including dyslipidemia, diabetes, smoking, and hypertension. Further, systemic inflammation is associated with oxidative stress increase and induces endothelial damage and atherosclerosis progression. Different miRNA have been already described in psoriasis, both in the skin tissues and in the blood flow, to play a role in the progression of disease. In this review, we will summarize and discuss the most important miRNAs that play a role in psoriasis and are also linked to CVD.
Keywords: cardiovascular diseases; microRNAs; psoriasis.
© The authors.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

