Type 2 immunity is maintained during cancer-associated adipose tissue wasting
- PMID: 34291232
- PMCID: PMC8286632
- DOI: 10.1093/immadv/ltab011
Type 2 immunity is maintained during cancer-associated adipose tissue wasting
Abstract
Objectives: Cachexia is a systemic metabolic disorder characterized by loss of fat and muscle mass, which disproportionately impacts patients with gastrointestinal malignancies such as pancreatic cancer. While the immunologic shifts contributing to the development of other adipose tissue (AT) pathologies such as obesity have been well described, the immune microenvironment has not been studied in the context of cachexia.
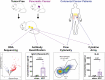
Methods: We performed bulk RNA-sequencing, cytokine arrays, and flow cytometry to characterize the immune landscape of visceral AT (VAT) in the setting of pancreatic and colorectal cancers.
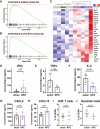
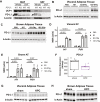
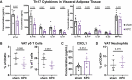
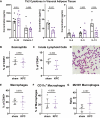
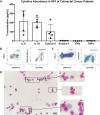
Results: The cachexia inducing factor IL-6 is strongly elevated in the wasting VAT of cancer bearing mice, but the regulatory type 2 immune landscape which characterizes healthy VAT is maintained. Pathologic skewing toward Th1 and Th17 inflammation is absent. Similarly, the VAT of patients with colorectal cancer is characterized by a Th2 signature with abundant IL-33 and eotaxin-2, albeit also with high levels of IL-6.
Conclusions: Wasting AT during the development of cachexia may not undergo drastic changes in immune composition like those seen in obese AT. Our approach provides a framework for future immunologic analyses of cancer associated cachexia.
Keywords: adipose tissue; cachexia; pancreatic cancer.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Immunology.
Figures


Similar articles
-
Adipose depot gene expression and intelectin-1 in the metabolic response to cancer and cachexia.J Cachexia Sarcopenia Muscle. 2020 Aug;11(4):1141-1153. doi: 10.1002/jcsm.12568. Epub 2020 Mar 31. J Cachexia Sarcopenia Muscle. 2020. PMID: 32232960 Free PMC article.
-
Abdominal fat and muscle distributions in different stages of colorectal cancer.BMC Cancer. 2023 Mar 28;23(1):279. doi: 10.1186/s12885-023-10736-2. BMC Cancer. 2023. PMID: 36978044 Free PMC article.
-
T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans.Arterioscler Thromb Vasc Biol. 2014 Dec;34(12):2637-43. doi: 10.1161/ATVBAHA.114.304636. Epub 2014 Oct 23. Arterioscler Thromb Vasc Biol. 2014. PMID: 25341798 Free PMC article. Clinical Trial.
-
Visceral adiposity and inflammatory bowel disease.Int J Colorectal Dis. 2021 Nov;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w. Epub 2021 Jun 9. Int J Colorectal Dis. 2021. PMID: 34104989 Review.
-
STAT3 in the systemic inflammation of cancer cachexia.Semin Cell Dev Biol. 2016 Jun;54:28-41. doi: 10.1016/j.semcdb.2016.02.009. Epub 2016 Feb 6. Semin Cell Dev Biol. 2016. PMID: 26860754 Free PMC article. Review.
Cited by
-
Metabolic Reprogramming in Adipose Tissue During Cancer Cachexia.Front Oncol. 2022 May 12;12:848394. doi: 10.3389/fonc.2022.848394. eCollection 2022. Front Oncol. 2022. PMID: 35646636 Free PMC article. Review.
-
Editorial: Immunology of cachexia.Front Immunol. 2023 Dec 5;14:1339263. doi: 10.3389/fimmu.2023.1339263. eCollection 2023. Front Immunol. 2023. PMID: 38116001 Free PMC article. No abstract available.
-
Blockade of innate inflammatory cytokines TNFα, IL-1β, or IL-6 overcomes virotherapy-induced cancer equilibrium to promote tumor regression.Immunother Adv. 2023 Jul 3;3(1):ltad011. doi: 10.1093/immadv/ltad011. eCollection 2023. Immunother Adv. 2023. PMID: 37461742 Free PMC article.
-
Cytokines in cancer.Cancer Cell. 2025 Jan 13;43(1):15-35. doi: 10.1016/j.ccell.2024.11.011. Epub 2024 Dec 12. Cancer Cell. 2025. PMID: 39672170 Free PMC article. Review.
-
cIAP1/2 Antagonism Induces Antigen-Specific T Cell-Dependent Immunity.J Immunol. 2023 Apr 1;210(7):991-1003. doi: 10.4049/jimmunol.2200646. J Immunol. 2023. PMID: 36881882 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
