The ERACE-PA Global Surveillance Program: Ceftolozane/tazobactam and Ceftazidime/avibactam in vitro Activity against a Global Collection of Carbapenem-resistant Pseudomonas aeruginosa
- PMID: 34291323
- PMCID: PMC8590662
- DOI: 10.1007/s10096-021-04308-0
The ERACE-PA Global Surveillance Program: Ceftolozane/tazobactam and Ceftazidime/avibactam in vitro Activity against a Global Collection of Carbapenem-resistant Pseudomonas aeruginosa
Abstract
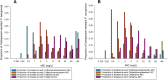
The cephalosporin-β-lactamase-inhibitor-combinations, ceftolozane/tazobactam and ceftazidime/avibactam, have revolutionized treatment of carbapenem-resistant Pseudomonas aeruginosa (CR-PA). A contemporary assessment of their in vitro potency against a global CR-PA collection and an assessment of carbapenemase diversity are warranted. Isolates determined as CR-PA by the submitting site were collected from 2019-2021 (17 centers in 12 countries) during the ERACE-PA Global Surveillance Program. Broth microdilution MICs were assessed per CLSI standards for ceftolozane/tazobactam, ceftazidime/avibactam, ceftazidime, and cefepime. Phenotypic carbapenemase testing was conducted (modified carbapenem inactivation method (mCIM)). mCIM positive isolates underwent genotypic carbapenemase testing using the CarbaR, the CarbaR NxG, or whole genome sequencing. The MIC50/90 was reported as well as percent susceptible (CLSI and EUCAST interpretation). Of the 807 isolates, 265 (33%) tested carbapenemase-positive phenotypically. Of these, 228 (86%) were genotypically positive for a carbapenemase with the most common being VIM followed by GES. In the entire cohort of CR-PA, ceftolozane/tazobactam and ceftazidime/avibactam had MIC50/90 values of 2/ > 64 and 4/64 mg/L, respectively. Ceftazidime/avibactam was the most active agent with 72% susceptibility per CLSI compared with 63% for ceftolozane/tazobactam. For comparison, 46% of CR-PA were susceptible to ceftazidime and cefepime. Against carbapenemase-negative isolates, 88 and 91% of isolates were susceptible to ceftolozane/tazobactam and ceftazidime/avibactam, respectively. Ceftolozane/tazobactam and ceftazidime/avibactam remained highly active against carbapenem-resistant P. aeruginosa, particularly in the absence of carbapenemases. The contemporary ERACE-PA Global Program cohort with 33% carbapenemase positivity including diverse enzymology will be useful to assess therapeutic options in these clinically challenging organisms with limited therapies.
Keywords: Carbapenem-resistant P. aeruginosa; Carbapenemase; Ceftazidime/avibactam; Ceftolozane/tazobactam.
© 2021. The Author(s).
Conflict of interest statement
A.B. is a speaker bureau member of Merck, Pfizer, and has received research support from FIND. H.S. has received grants or research support from the German Research Foundation (DFG) and the German Centre for Infection Research (DZIF). H.S. is a consultant or speaker bureau member for Basilea, Entasis, Eumedica, Gilead, MSD, and Shionogi. D.P.N. is a consultant, speaker bureau member or has received research support from Abbvie, Cepheid, Merck, Paratek, Pfizer, Wockhardt, Shionogi, and Tetraphase. All other authors non to declare.
Figures
References
-
- Zerbaxa® (2020) (ceftolozane/tazobactam) [Package Insert]. Merck Sharpe & Dohme Corp., Whitehouse Station. https://www.merck.com/product/usa/pi_circulars/z/zerbaxa/zerbaxa_pi.pdf
-
- Avycaz® (2020) (ceftazidime/avibactam) [Package Insert]. Allergan USA, Inc., Madison. https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/...
-
- Grupper M, Sutherland C, Nicolau DP. Multicenter evaluation of ceftazidime-avibactam and ceftolozane-tazobactam inhibitory activity against meropenem-nonsusceptible pseudomonas aeruginosa from blood, respiratory tract, and wounds. Antimicrob Agents Chemother. 2017;61:e00875–e917. doi: 10.1128/AAC.00875-17. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

