Dynamic regulation of mitotic ubiquitin ligase APC/C by coordinated Plx1 kinase and PP2A phosphatase action on a flexible Apc1 loop
- PMID: 34291488
- PMCID: PMC8441438
- DOI: 10.15252/embj.2020107516
Dynamic regulation of mitotic ubiquitin ligase APC/C by coordinated Plx1 kinase and PP2A phosphatase action on a flexible Apc1 loop
Abstract
The anaphase-promoting complex/cyclosome (APC/C), a multi-subunit ubiquitin ligase essential for cell cycle control, is regulated by reversible phosphorylation. APC/C phosphorylation by cyclin-dependent kinase 1 (Cdk1) promotes Cdc20 co-activator loading in mitosis to form active APC/C-Cdc20. However, detailed phospho-regulation of APC/C dynamics through other kinases and phosphatases is still poorly understood. Here, we show that an interplay between polo-like kinase (Plx1) and PP2A-B56 phosphatase on a flexible loop domain of the subunit Apc1 (Apc1-loop500 ) controls APC/C activity and mitotic progression. Plx1 directly binds to the Apc1-loop500 in a phosphorylation-dependent manner and promotes the formation of APC/C-Cdc20 via Apc3 phosphorylation. Upon phosphorylation of loop residue T532, PP2A-B56 is recruited to the Apc1-loop500 and differentially promotes dissociation of Plx1 and PP2A-B56 through dephosphorylation of Plx1-binding sites. Stable Plx1 binding, which prevents PP2A-B56 recruitment, prematurely activates the APC/C and delays APC/C dephosphorylation during mitotic exit. Furthermore, the phosphorylation status of the Apc1-loop500 is controlled by distant Apc3-loop phosphorylation. Our study suggests that phosphorylation-dependent feedback regulation through flexible loop domains within a macromolecular complex coordinates the activity and dynamics of the APC/C during the cell cycle.
Keywords: APC/C; Cdc20; PP2A; loop domain; polo-like kinase.
© 2021 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
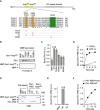
Schematic diagram of Apc1 is shown. Two loop domains (loop300, 294–399; loop500, 515–584) and PC repeat domain are shown in yellow and in cyan, respectively. Multiple alignment of sequences of Apc1‐loop500 containing PBMs (Orange) and B56‐binding site (green) is shown. Introduced alanine substitutions are indicated as A in red. Hs, Homo sapiens human; Pt, Pan troglodytes chimpanzee; Mm, Mus musculus mouse; Gg, Gallus gallus chicken; and Xt, Xenopus tropicalis frog. Conserved or similar amino acids are shown with an asterisk (*) or dot (.), respectively.
Binding assay using MBP‐fused Apc1‐loop500 fragments. MBP‐fused Apc1‐loop500 WT or its derivatives (T532A, T539A, 2A (T532A/T539A) and S558A) was incubated with anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h. The bound proteins were recovered by amylose beads, separated by SDS–PAGE and detected by immunoblotting with Plx1 antibody and Ponceau S staining.
Quantification of (B). The bar graph is quantification of bound Plx1. The intensities of Apc1‐loop500 WT were arbitrarily set to 1.0. Error bars, SEM from three independent experiments.
Specific binding of Plx1‐PBD to Apc1‐loop500. MBP‐fused Apc1‐loop500 WT or its derivatives 2A (T532A/T539A) was incubated in anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h and further incubated in the presence of 10 µg of WT Plx1‐PBD for 15 min. The bound proteins were recovered by amylose beads, separated by SDS–PAGE and detected by Coomassie brilliant blue staining (CBB). Purified proteins (Plx1‐PBD and Apc1‐loop500) were run as a standard.
Quantification of (D). The bar graph is quantification of bound Plx1‐PBD. The intensities of WT were arbitrarily set to 1.0. Error bars, SEM from three independent experiments.
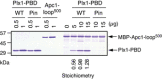
Plx1‐PBD, but not its Pincer mutant, binds to Apc1‐loop500. MBP‐fused Apc1‐loop500 WT was incubated in anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h and further incubated in the presence of indicated amounts of WT Plx1‐PBD or Plx1‐PBD Pincer mutant (Pin) for 15 min. The bound proteins were recovered by amylose beads and analysed as described in (D). The bound Plx1‐PBD is shown as stoichiometries (the bound Plx1‐PBD per Apc1‐loop500) determined from Fig EV1.
CDK‐dependent binding of Plx1‐PBD to the Apc1‐loop500. MBP‐fused Apc1‐loop500 was incubated in the presence or absence of purified Cdk2/cyclin A at 30°C for 2 h. MBP‐fused Apc1‐loop500 (CDK+/−) was isolated by amylose beads and further incubated with indicated amounts of WT Plx1‐PBD on ice for 10 min. The bound proteins were recovered by amylose beads and analysed as described in (D).

Cdc20‐binding assay in Xenopus egg extracts. The purified recombinant WT or Apc‐loop500 mutant APC/Cs with mutations (1‐T532A, 1‐T539A, 1‐T532A/T539A (1–2A) and 1‐S558A) was incubated with APC/C‐depleted (ΔAPC) interphase extract (Inter) or ΔAPC anaphase extracts supplemented with non‐degradable cyclin B (Ana) at 23°C for 1 h. The APC/C was recovered with Apc3 monoclonal antibody (AF3.1) beads, and the bound proteins were analysed by SDS–PAGE and immunoblotting with indicated antibodies.
Quantification of (A). The bar graph is quantification of bound Cdc20. The intensities of WT control in anaphase were arbitrarily set to 1.0. Error bars, SEM from three independent experiments.
Plx1‐binding‐deficient APC/Cs are less active in ubiquitylation assay than WT APC/C. The purified recombinant WT APC/C or mutant APC/C (1‐2A or 1‐S558A) was incubated with ΔAPC anaphase extract and the recovered APC/C‐Cdc20 complex was subjected to ubiquitylation assay (Appendix Fig S3). Ubiquitylation was quantified, and the activity of WT at 45 min was arbitrarily set to 1.0. Error bars, SEM from three independent experiments. A representative result is shown in Appendix Fig S3.
In vitro kinase assay of Apc3‐loop using purified Plx1. The purified Apc3‐loop (182–451 in Xenopus) fragment was incubated with Plx1 in the presence of ATP at 23°C for indicated times, separated by SDS–PAGE and detected by Coomassie brilliant blue (CBB).
In vitro phosphorylation by Plx1. Apc3‐loop fragment, α‐Casein, N‐terminus of Cdc20 (N159) or MBP protein was incubated with purified Plx1 in the presence of [γ32P]‐ATP at 23°C for indicated times, separated by SDS–PAGE and detected by autoradiography and Coomassie brilliant blue (CBB) staining.
Phosphorylation of Apc3‐loop by Plx1 in the presence of [γ32P]‐ATP. WT Apc3‐loop fragment or its CDK site mutant (9A) was incubated with purified Plx1 in the presence of [γ32P]‐ATP at 23°C for indicated times, separated by SDS–PAGE and detected by autoradiography.

Cdc20‐dependent destruction assay in anaphase. The purified recombinant WT APC/C or its derivatives (1–2A and 1‐S558A) was incubated with its substrates (35S‐labelled cyclin B and a version of cyclin B lacking the N‐terminal 67 residues, Δ67) in APC/C‐depleted (ΔAPC) interphase extracts supplemented with non‐degradable cyclin B at 23°C. Samples taken at indicated time points were analysed by SDS–PAGE and autoradiography.
Quantification of (A). The relative cyclin levels are shown, normalised with reference to the intensities found at time 0 for each time point. Error bars, SEM from three independent experiments.
Cdh1‐dependent destruction assay. The purified recombinant WT APC/C or its derivatives (1–2A and 1‐S558A) was incubated with its substrates in APC/C‐depleted (ΔAPC) interphase extracts supplemented with Cdh1 at 23°C. Samples taken at indicated time points were analysed by SDS–PAGE and autoradiography.
Quantification of (C). The relative cyclin levels are shown, normalised with reference to the intensities found at time 0 for each time point. Error bars, SEM from three independent experiments.

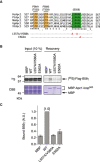
Apc1‐loop500 is the hub for both Plx1 and PP2A‐B56 phosphatase. CDK sites (T or S) and B56‐binding site (green) on Apc1‐loop500 (yellow bar) are shown. Bindings of Plx1 and B56 to Apc1‐loop500 depend on phosphorylation of CDK sites. The PP2A‐B56 associated with Apc1‐loop500 may dephosphorylate CDK sites.
Phosphorylation status of T532 and T539 in Apc1‐loop500 varies depending on mutations in the B56‐binding site. MBP‐fused Apc1‐loop500 WT or its derivatives (L557A/V560A and E562A) was incubated with anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h. The proteins were recovered by amylose beads, separated by SDS–PAGE and detected by immunoblotting with phospho‐specific (pAb) or MBP antibody.
Plx1‐binding assay using MBP‐fused Apc1‐loop500 fragments with mutations in B56‐binding site. The Plx1 binding to Apc1‐loop500 WT or its derivatives (L557A/V560A or E562A) was analysed as described in Fig 1B.
Quantification of (C). The bar graph is quantification of bound Plx1. The intensities of WT were arbitrarily set to 1.0. Error bars, SEM from three independent experiments.
Binding assay using MBP‐fused Apc1‐loop500 fragments and B56γ MBP‐fused Apc1‐loop500 WT or its derivatives (T532A, T539A, T532A/T539A (2A) and S558A) was incubated with the 35S‐labelled Flag‐B56γ in anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h. The bound proteins were recovered by amylose beads, separated by SDS–PAGE and detected by autoradiography or Coomassie brilliant blue (CBB) staining. The recovery of WT (×1/2 and ×1) was run as a standard.
Quantification of (E). The bar graph is quantification of bound B56γ. The intensities of WT were arbitrarily set to 1.0. Error bars, SEM from three independent experiments.
Summary of Plx1 and B56 binding to Apc1‐loop500 derivatives. The abilities of Apc1‐loop500 fragments carrying the indicated mutations are shown. The B56 bindings to ∆11 (deletion of 11 residues including B56‐binding site) and 3A (T532A/T539A/S558A) were tested previously (Fujimitsu & Yamano, 2020).
B56‐binding site mutations (L557A/V560A and E562A) were shown in multiple alignment of sequences of Apc1‐loop500 as Fig 1A.
Binding assay using MBP‐fused Apc1‐loop500 fragments and B56γ. MBP‐fused Apc1‐loop500 WT or its derivatives (L557A/V560A or E562A) was incubated with the 35S‐labelled Flag‐B56γ in anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h. The bound proteins were recovered by amylose beads, separated by SDS–PAGE and detected by autoradiography or Coomassie brilliant blue (CBB) staining.
Quantification of (B). The bar graph is quantification of bound B56γ. The intensities of WT were arbitrarily set to 1.0. Error bars, SEM from three independent experiments.
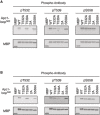
MBP‐fused Apc1‐loop500 WT or its derivatives (T532A, T539A, 2A and S558A) was incubated with anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h. The bound proteins were recovered by amylose beads, separated by SDS–PAGE and detected by immunoblotting with a phospho‐specific antibody (pT532, pT539 or pS558) and MBP antibody.
Phospho‐specific antibodies recognise CDK sites of Apc1‐loop500 phosphorylated by Cdk2/cyclin A in vitro. MBP‐fused Apc1‐loop500 WT and its derivatives (T532A, T539A, 2A and S558A) recovered in Appendix Fig S2 were analysed as described in A.

Plx1 and PP2A‐B56 competes for Apc1‐loop500. MBP‐fused Apc1‐loop500 WT or its derivatives (∆11, deletion of 11 residues including the B56‐binding motif) was incubated with the 35S‐labelled Flag‐B56γ in anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h and further incubated with indicated amounts of WT Plx1‐PBD or Plx1‐PBD Pincer mutant (Pin) for 15 min. The bound proteins were recovered by amylose beads, separated by SDS–PAGE and detected by autoradiography or Coomassie brilliant blue (CBB) staining.
Dephosphorylation of T532, T539 and S558 on Apc1 by PP2A‐B56. MBP‐fused Apc1‐loop500 WT or its derivatives (L557A/V560A and E562A) was incubated in anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h, isolated by amylose beads and further incubated in the presence (78 nM) or absence of purified PP2A‐B56γ at 23°C for 20 min. The proteins were recovered by amylose beads, separated by SDS–PAGE and detected by immunoblotting with phospho‐specific or MBP antibodies. The signals of a phospho‐specific antibody and MBP antibody on the same membrane were detected in the 800‐ and 700‐nm channels, respectively. The two channels were pseudo‐coloured (green; pT532, pT539 and pS558, red; MBP) and overlaid in the bottom panel (Overlay). The signal of pS558 was very faint in L557A/V560A mutant probably because these two residue are close to S558, interfering with pS558 antibody recognition.
Dephosphorylation of Apc1‐loop500 by PP2A‐B56 in the presence of Plx1‐PBD. MBP‐fused Apc1‐loop500 WT or its derivatives (L557A/V560A or E562A) was incubated in anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h and further incubated with 10 µg of WT Plx1‐PBD for 15 min. The complexes were isolated by amylose beads and incubated in the presence (78 nM) or absence of purified PP2A‐ B56γ at 23°C for 20 min. The proteins were recovered by amylose beads and analysed as described in (B).

Phosphorylation kinetics of T532, T539 and S558 on Apc1‐loop500 in anaphase extracts. MBP‐fused Apc1‐loop500 WT or its derivatives (∆11, deletion of 11 residues including the B56‐binding motif) was incubated with Xenopus egg extracts in the presence of non‐degradable cyclin B at 23°C. The samples taken as indicated were analysed by SDS–PAGE and immunoblotting using phospho‐specific antibodies (pT532, pT539 and pS558) or MBP antibody. To monitor CDK activity, Apc3 antibody was used (bottom). The signals of a phospho‐specific antibody and MBP antibody on the same membrane were detected in the 800‐ and 700‐nm channels, respectively. The two channels were pseudo‐coloured (green; pT532, pT539 and pS558, red; MBP) and overlaid in the bottom panel (Overlay). The signal of pS558 was not detected in ∆11 mutant because ∆11 mutation includes S558.
Quantification of (A). The intensities of WT at 50 min were arbitrarily set to 1.0. Error bars, SEM from three independent experiments.
Phosphorylation kinetics of T532, T539 and S558 on Apc1‐loop500 of the endogenous APC/C in Xenopus egg extract. Endogenous APC/C was immunoprecipitated with Apc3 MAb (AF3.1) beads at the indicated time points after mitotic induction by adding non‐degradable cyclin B and analysed by SDS–PAGE and immunoblotting with phospho‐specific antibodies, pT532, pT539 and pS558. Quantification of Fig EV5A. The intensities at 45 min were arbitrarily set to 1.0. Error bars, SEM from three independent experiments.
Dissociation of Plx1‐PBD from Apc1‐loop500. MBP‐fused Apc1‐loop500 WT or its derivatives (E562A) was incubated in anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h and further incubated with 10 µg of WT Plx1‐PBD for 15 min. The complexes were isolated by amylose beads and incubated in the presence (78 nM) or absence of purified PP2A‐B56γ at 23°C for 30 or 60 min. The bound proteins were recovered by amylose beads, separated by SDS–PAGE and detected by Coomassie brilliant blue (CBB) staining. The intensities at 0 min were arbitrarily set to 1.0.

Endogenous APC/C was immunoprecipitated with Apc3 monoclonal antibody (AF3.1) beads after incubation at 23°C for the indicated time points after mitotic induction by adding non‐degradable cyclin B∆167. The bound proteins were analysed by SDS–PAGE and immunoblotting with indicated antibodies. See Fig 5C for quantification. “Inter” denotes interphase.
Monitoring of mitotic induction from interphase extract upon cyclin B addition. When Cdk1 becomes active, Apc3 is hyper‐phosphorylated, as observed with the band shifts of Apc3. Samples were taken at the indicated times and analysed by SDS–PAGE and immunoblotting with indicated antibodies.
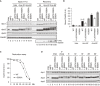
Cdc20‐binding assay in Xenopus egg extracts. The Cdc20‐binding activities of WT APC/C and Apc‐loop500‐mutant APC/Cs with mutations (1‐L557A/V560A or 1‐E562A) were analysed as described in Fig 2A.
Quantification of (A). The bar graph is quantification of bound Cdc20.The intensities of WT control at Ana‐45 min were arbitrarily set to 1.0. Error bars, SEM from three independent experiments.
Premature activation of a mutant APC/C (1‐E562A) in Xenopus egg extracts. The purified recombinant WT APC/C or Apc‐loop500‐mutant APC/C (1‐E562A) was incubated with its substrates (35S‐labelled cyclin B and a version of cyclin B lacking the N‐terminal 67 residues, ∆67) in APC/C‐depleted (ΔAPC) interphase extracts supplemented with non‐degradable cyclin B at 23°C. Samples taken at indicated time points after addition of substrates were analysed by SDS–PAGE and autoradiography (Appendix Fig S7A). The relative cyclin levels are shown, normalised with reference to the intensities found at time 0 for each time point. Error bars, SEM from three independent experiments. A representative result is shown in Appendix Fig S7A.
APC/C dephosphorylation after inactivation of CDK. The purified recombinant WT or Apc‐loop500‐mutant APC/Cs with mutations (1‐L557A/V560A or 1‐E562A) was incubated with APC/C‐depleted (ΔAPC) in anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 1 h (Ana). After addition of p27 (0.3 µM), the samples taken at time points shown were analysed by SDS–PAGE and immunoblotting with indicated antibodies. “Inter” denotes interphase.
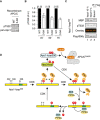
The mutations in CDK sites on Apc3‐loop reduces phosphorylation of T532 on Apc1. The purified recombinant WT or a mutant APC/C with mutations at nine CDK sites in Apc3‐loop (3–9A) was incubated with APC/C‐depleted (ΔAPC) anaphase extracts supplemented with non‐degradable cyclin B at 23°C for 60 min. The APC/C was recovered with Apc3 monoclonal antibody (AF3.1) beads, and the phosphorylation status of T532 in Apc1 was analysed by SDS–PAGE and immunoblotting with phospho‐specific (pT532) or Apc1 (pan‐Apc1) antibodies.
Similar to (A), the experiments were performed at three time points, 45, 60 or 75 min in anaphase extracts. Quantification of phosphorylation levels of T532 is shown. The intensities of pT532 signals in WT at each time point (45, 60 or 75 min) were arbitrarily set to 1.0. The level of T532‐phosphorylation was normalised to pan‐Apc1. Error bars, SEM from three independent experiments.
PP2A‐B56 preferentially binds to T532‐phoshorylated Apc1‐loop500. Flag‐tagged B56γ was incubated with the MBP‐fused Apc1‐loop500 phosphorylated by Cdk2/cyclin A. The bound proteins were recovered by anti‐flag affinity M2 beads, separated by SDS–PAGE and detected by immunoblotting with phospho‐specific (pT532), MBP or Flag antibody. The signals of a phospho‐specific antibody (pT532) and MBP were pseudo‐coloured (green; pT532, red; MBP) and overlaid (Overlay).
A feedback control of APC/C by Plx1 and PP2A‐B56 on Apc1‐loop500. CDK phosphorylates Apc1‐loop500 at T539 and S558 and promotes Plx1 binding onto Apc1‐loop500 via its PBD. Two PBD interactions, possibly dimerisation of Plx1, may stabilise the complex between Plx1 and Apc1‐loop500. Plx1 binding blocks PP2A‐B56 recruitment to Apc1‐loop500 and stimulates Apc3‐loop phosphorylation, which may induce Cdk1‐CyclinB‐p9/Cks2 recruitment and subsequent Apc1‐loop300 phosphorylation. Cdk1 recruited onto phosphorylated Apc3‐loop promotes phosphorylation of T532, a residue important for the recruitment of PP2A‐B56 on Apc1‐loop500. PP2A‐B56 recruitment promotes dissociation of Plx1 by competitive binding and by dephosphorylation of T532 and T539, which diminishes Plx1‐mediated phosphorylation of Apc3‐loop. PP2A‐B56 may quickly dissociate from Apc1‐loop500 once T532 is dephosphorylated, but Apc1‐loop500 is phosphorylated again by Cdk1 while Cdk1 is active. The dynamic phosphorylation–dephosphorylation cycle may continue until Cdk1 is inactivated, allowing swift response of the APC/C during the cell cycle.
Similar articles
-
PP2A-B56 binds to Apc1 and promotes Cdc20 association with the APC/C ubiquitin ligase in mitosis.EMBO Rep. 2020 Jan 7;21(1):e48503. doi: 10.15252/embr.201948503. Epub 2019 Dec 11. EMBO Rep. 2020. PMID: 31825153 Free PMC article.
-
CDK1-PP2A-B55 interplay ensures cell cycle oscillation via Apc1-loop300.Cell Rep. 2024 May 28;43(5):114155. doi: 10.1016/j.celrep.2024.114155. Epub 2024 Apr 27. Cell Rep. 2024. PMID: 38678563
-
The PP2AB56 phosphatase promotes the association of Cdc20 with APC/C in mitosis.J Cell Sci. 2017 May 15;130(10):1760-1771. doi: 10.1242/jcs.201608. Epub 2017 Apr 12. J Cell Sci. 2017. PMID: 28404789 Free PMC article.
-
Getting out of mitosis: spatial and temporal control of mitotic exit and cytokinesis by PP1 and PP2A.FEBS Lett. 2019 Oct;593(20):2908-2924. doi: 10.1002/1873-3468.13595. Epub 2019 Sep 18. FEBS Lett. 2019. PMID: 31494926 Review.
-
Xenopus Polo-like kinase Plx1: a multifunctional mitotic kinase.Oncogene. 2005 Jan 10;24(2):238-47. doi: 10.1038/sj.onc.1208220. Oncogene. 2005. PMID: 15640839 Review.
Cited by
-
A bifunctional kinase-phosphatase module balances mitotic checkpoint strength and kinetochore-microtubule attachment stability.EMBO J. 2023 Oct 16;42(20):e112630. doi: 10.15252/embj.2022112630. Epub 2023 Sep 15. EMBO J. 2023. PMID: 37712330 Free PMC article.
-
A force-sensitive mutation reveals a non-canonical role for dynein in anaphase progression.J Cell Biol. 2024 Oct 7;223(10):e202310022. doi: 10.1083/jcb.202310022. Epub 2024 Jul 1. J Cell Biol. 2024. PMID: 38949648 Free PMC article.
-
Spatial control of the APC/C ensures the rapid degradation of cyclin B1.EMBO J. 2024 Oct;43(19):4324-4355. doi: 10.1038/s44318-024-00194-2. Epub 2024 Aug 14. EMBO J. 2024. PMID: 39143240 Free PMC article.
References
-
- Archambault V, Glover DM (2009) Polo‐like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol 10: 265–275 - PubMed
-
- Berger I, Fitzgerald DJ, Richmond TJ (2004) Baculovirus expression system for heterologous multiprotein complexes. Nat Biotechnol 22: 1583–1587 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

