Brain perfusion magnetic resonance imaging using pseudocontinuous arterial spin labeling in 314 dogs and cats
- PMID: 34291497
- PMCID: PMC8478041
- DOI: 10.1111/jvim.16215
Brain perfusion magnetic resonance imaging using pseudocontinuous arterial spin labeling in 314 dogs and cats
Abstract
Background: Arterial spin labeling (ASL) is a noninvasive brain perfusion magnetic resonance imaging (MRI) technique that has not been assessed in clinical veterinary medicine.
Hypothesis/objectives: To test the feasibility of ASL using a 1.5 Tesla scanner and provide recommendations for optimal quantification of cerebral blood flow (CBF) in dogs and cats.
Animals: Three hundred fourteen prospectively selected client-owned dogs and cats.
Methods: Each animal underwent brain MRI including morphological sequences and ≥1 ASL sequences using different sites of blood labeling and postlabeling delays (PLD). Calculated ASL success rates were compared. The CBF was quantified in animals that had morphologically normal brain MRI results and parameters of ASL optimization were investigated.
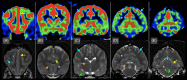
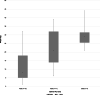
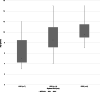
Results: Arterial spin labeling was easily implemented with an overall success rate of 95% in animals with normal brain MRI. Technical recommendations included (a) positioning of the imaging slab at the foramen magnum and (b) selected PLD of 1025 ms in cats and dogs <7 kg, 1525 ms in dogs 7 to 38 kg, and 2025 ms in dogs >38 kg. In 37 dogs, median optimal CBF in the cortex and thalamic nuclei were 114 and 95 mL/100 g/min, respectively. In 28 cats, median CBF in the cortex and thalamic nuclei were 113 and 114 mL/100 g/min, respectively.
Conclusions and clinical importance: Our survey of brain perfusion ASL-MRI demonstrated the feasibility of ASL at 1.5 Tesla, suggested technical recommendations and provided CBF values that should be helpful in the characterization of various brain diseases in dogs and cats.
Keywords: ASL; MRI; arterial spin labeling; brain; cat; dog; perfusion.
© 2021 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Authors declare no conflict of interest.
Figures



References
-
- Buck J, Larkin JR, Simard MA, Khrapitchev AA, Chappell MA, Sibson NR. Sensitivity of multiphase pseudocontinuous arterial spin labelling (MP pCASL) magnetic resonance imaging for measuring brain and tumour blood flow in mice. Contrast Media Mol Imaging. 2018;7:ID4580919. 10.1155/2018/4580919. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
