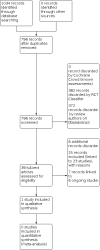
Interventions for the prevention of persistent post-COVID-19 olfactory dysfunction
- PMID: 34291812
- PMCID: PMC8406518
- DOI: 10.1002/14651858.CD013877.pub2
Interventions for the prevention of persistent post-COVID-19 olfactory dysfunction
Update in
-
Interventions for the prevention of persistent post-COVID-19 olfactory dysfunction.Cochrane Database Syst Rev. 2022 Sep 5;9(9):CD013877. doi: 10.1002/14651858.CD013877.pub3. Cochrane Database Syst Rev. 2022. PMID: 36063364 Free PMC article.
Abstract
Background: Loss of olfactory function is well recognised as a cardinal symptom of COVID-19 infection, and the ongoing pandemic has resulted in a large number of affected individuals with abnormalities in their sense of smell. For many, the condition is temporary and resolves within two to four weeks. However, in a significant minority the symptoms persist. At present, it is not known whether early intervention with any form of treatment (such as medication or olfactory training) can promote recovery and prevent persisting olfactory disturbance. OBJECTIVES: To assess the effects (benefits and harms) of interventions that have been used, or proposed, to prevent persisting olfactory dysfunction due to COVID-19 infection. A secondary objective is to keep the evidence up-to-date, using a living systematic review approach. SEARCH METHODS: The Cochrane ENT Information Specialist searched the Cochrane COVID-19 Study Register; Cochrane ENT Register; CENTRAL; Ovid MEDLINE; Ovid Embase; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished studies. The date of the search was 16 December 2020.
Selection criteria: Randomised controlled trials including participants who had symptoms of olfactory disturbance following COVID-19 infection. Individuals who had symptoms for less than four weeks were included in this review. Studies compared any intervention with no treatment or placebo. DATA COLLECTION AND ANALYSIS: We used standard Cochrane methodological procedures. Our primary outcomes were the presence of normal olfactory function, serious adverse effects and change in sense of smell. Secondary outcomes were the prevalence of parosmia, change in sense of taste, disease-related quality of life and other adverse effects (including nosebleeds/bloody discharge). We used GRADE to assess the certainty of the evidence for each outcome. MAIN RESULTS: We included one study of 100 participants, which compared an intranasal steroid spray to no intervention. Participants in both groups were also advised to undertake olfactory training for the duration of the trial. Data were identified for only two of the prespecified outcomes for this review, and no data were available for the primary outcome of serious adverse effects. Intranasal corticosteroids compared to no intervention (all using olfactory training) Presence of normal olfactory function after three weeks of treatment was self-assessed by the participants, using a visual analogue scale (range 0 to 10, higher scores = better). A score of 10 represented "completely normal smell sensation". The evidence is very uncertain about the effect of intranasal corticosteroids on self-rated recovery of sense of smell (estimated absolute effect 619 per 1000 compared to 520 per 1000, risk ratio (RR) 1.19, 95% confidence interval (CI) 0.85 to 1.68; 1 study; 100 participants; very low-certainty evidence). Change in sense of smell was not reported, but the self-rated score for sense of smell was reported at the endpoint of the study with the same visual analogue scale (after three weeks of treatment). The median scores at endpoint were 10 (interquartile range (IQR) 9 to 10) for the group receiving intranasal corticosteroids, and 10 (IQR 5 to 10) for the group receiving no intervention (1 study; 100 participants; very low-certainty evidence).
Authors' conclusions: There is very limited evidence regarding the efficacy of different interventions at preventing persistent olfactory dysfunction following COVID-19 infection. However, we have identified a small number of additional ongoing studies in this area. As this is a living systematic review, the evidence will be updated regularly to incorporate new data from these, and other relevant studies, as they become available. For this (first) version of the living review, we identified a single study of intranasal corticosteroids to include in this review, which provided data for only two of our prespecified outcomes. The evidence was of very low certainty, therefore we were unable to determine whether intranasal corticosteroids may have a beneficial or harmful effect.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Katie Webster: none known.
Lisa O' Byrne: none known.
Samuel MacKeith: Sam MacKeith is Assistant Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Carl Philpott: Professor Carl Philpott sees and treats patients with COVID‐19 related smell loss. He has written various online publications on the topic and conducted interviews and webinars internationally. He is a Trustee for the charity Fifth Sense. He is the senior author on the Clinical Olfactory Working Group consensus document on the management of post‐infectious olfactory dysfunction and the consensus document on the use of systemic corticosteroids in COVID‐19 related olfactory dysfunction.
Claire Hopkins: Professor Claire Hopkins sees and treats patients with COVID‐19 related smell loss. She has spoken on the association between COVID and smell loss in multiple media outlets. She is senior author of the British Rhinological Society position paper on management of COVID‐19 related smell loss.
Martin Burton: Professor Martin Burton is Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Figures
Similar articles
-
Interventions for the treatment of persistent post-COVID-19 olfactory dysfunction.Cochrane Database Syst Rev. 2021 Jul 22;7(7):CD013876. doi: 10.1002/14651858.CD013876.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Sep 5;9:CD013876. doi: 10.1002/14651858.CD013876.pub3. PMID: 34291813 Free PMC article. Updated.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Intranasal corticosteroids for non-allergic rhinitis.Cochrane Database Syst Rev. 2019 Nov 2;2019(11):CD010592. doi: 10.1002/14651858.CD010592.pub2. Cochrane Database Syst Rev. 2019. PMID: 31677153 Free PMC article.
-
Intratympanic corticosteroids for Ménière's disease.Cochrane Database Syst Rev. 2023 Feb 27;2(2):CD015245. doi: 10.1002/14651858.CD015245.pub2. Cochrane Database Syst Rev. 2023. PMID: 36847608 Free PMC article. Review.
-
Biologics for chronic rhinosinusitis.Cochrane Database Syst Rev. 2020 Feb 27;2(2):CD013513. doi: 10.1002/14651858.CD013513.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 12;3:CD013513. doi: 10.1002/14651858.CD013513.pub3. PMID: 32102112 Free PMC article. Updated.
Cited by
-
Use of platelet-rich plasma for COVID-19-related olfactory loss: a randomized controlled trial.Int Forum Allergy Rhinol. 2023 Jun;13(6):989-997. doi: 10.1002/alr.23116. Epub 2022 Dec 21. Int Forum Allergy Rhinol. 2023. PMID: 36507615 Free PMC article. Clinical Trial.
-
Characteristics of Living Systematic Review for COVID-19.Clin Epidemiol. 2022 Aug 4;14:925-935. doi: 10.2147/CLEP.S367339. eCollection 2022. Clin Epidemiol. 2022. PMID: 35958161 Free PMC article.
-
Störungen des Riech- und Schmeckvermögens bei COVID-19.Allergo J. 2022;31(7):35-43. doi: 10.1007/s15007-022-5602-x. Epub 2022 Oct 31. Allergo J. 2022. PMID: 36339652 Free PMC article. Review. German.
-
Recovery rates and long-term olfactory dysfunction following COVID-19 infection.World J Otorhinolaryngol Head Neck Surg. 2024 Mar 19;10(2):121-128. doi: 10.1002/wjo2.163. eCollection 2024 Jun. World J Otorhinolaryngol Head Neck Surg. 2024. PMID: 38855291 Free PMC article. Review.
-
Olfactory and gustatory disorders in COVID-19.Allergo J Int. 2022;31(7):243-250. doi: 10.1007/s40629-022-00216-7. Epub 2022 Jun 20. Allergo J Int. 2022. PMID: 35755859 Free PMC article. Review.
References
References to studies included in this review
Abdelalim 2021 {published data only}
-
- NCT04484493. Corticosteroid nasal spray in COVID-19 anosmia [Role of corticosteroid nasal spray in recovery of smell sensation in COVID-19 patients]. https://clinicaltrials.gov/show/NCT04484493 (first received 23 July 2020). [CENTRAL: CN-02145535]
References to studies excluded from this review
ACTION (NCT04332107) {published data only}
-
- NCT04332107. Azithromycin for COVID-19 treatment in outpatients nationwide [Azithromycin for prevention of disease progression in patients with mild or moderate COVID-19]. https://clinicaltrials.gov/show/NCT04332107 (first received 2 April 2020).
Begam 2020 {published data only}
COPPS (NCT04662060) {published data only}
-
- NCT04662060. COVID-19 Outpatient Pragmatic Platform Study (COPPS) - Acebilustat Sub-Protocol [COVID-19 Outpatient Pragmatic Platform Study (COPPS): a pragmatic multi-arm, adaptive, phase 2, blinded, randomized placebo-controlled platform trial to assess the efficacy of different investigational therapeutics in reducing time to disease resolution or viral load cessation, as compared to standard supportive care in outpatients with COVID-19]. https://clinicaltrials.gov/show/NCT04662060 (first received 10 December 2020).
Co‐STAR (NCT04422275) {published data only}
-
- NCT04422275. Coronavirus smell therapy for anosmia recovery. https://clinicaltrials.gov/show/NCT04422275 (first received 9 June 2020). [CENTRAL: CN-02118715]
COVIDAtoZ (NCT04342728) {published data only}
-
- NCT04342728. Coronavirus 2019 (COVID-19)- using ascorbic acid and zinc supplementation [Coronavirus disease 2019- using ascorbic acid and zinc supplementation (COVIDAtoZ) research study a randomized, open label single center study]. https://clinicaltrials.gov/show/NCT04342728 (first received 13 April 2020).
COVIDORL (NCT04361474) {published data only}
-
- Daval M, Corre A, Palpacuer C, Housset J, Poillon G, Eliezer M, et al. Efficacy of local budesonide therapy in the management of persistent hyposmia in COVID-19 patients without signs of severity: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):666. [CENTRAL: CN-02177639] [EMBASE: 632388256] [PMID: ] - PMC - PubMed
-
- NCT04361474. Trial evaluating the efficacy of local budesonide therapy in the management of hyposmia in COVID-19 patients without signs of severity [A randomized controlled trial evaluating the efficacy of local budesonide therapy in the management of hyposmia in COVID-19 patients without signs of severity]. https://clinicaltrials.gov/show/NCT04361474 (first received 24 April 2020). [CENTRAL: CN-02180471] - PubMed
CTRI/2020/08/027477 {published data only}
-
- CTRI/2020/08/027477. Evaluation of safety and efficacy of T-AYU-HM Premium and Onion steam vaporization/nebulization in Covid-19 patients. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/08/027477 (first received 2020).
IRCT20200522047542N1 {published data only}
-
- IRCT20200522047542N1. Effect of Corton on olfactory dysfunction in COVID-19 patients [Effect of inhaled corticosteroids in the treatment of anosmia in patients with COVID-19]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20200522047542N1 (first received 2020). [CENTRAL: CN-02187839]
NCT04374474 {published data only}
-
- NCT04374474. Anosmia rehabilitation in patients post coronavirus disease (COVID 19) [Olfactory retraining therapy and budesonide nasal rinse for anosmia treatment in patients post-COVID 19. A randomized controlled trial]. https://clinicaltrials.gov/show/NCT04374474 (first received 5 May 2020). [CENTRAL: CN-02093904]
NCT04382547 {published data only}
-
- NCT04382547. Treatment of Covid-19 associated pneumonia with allogenic pooled olfactory mucosa-derived mesenchymal stem cells. https://clinicaltrials.gov/show/NCT04382547 (first received 11 May 2020).
NCT04406584 {published data only}
-
- NCT04406584. Intranasal Injection of PRP versus saline for treatment of olfactory dysfunction [Intranasal injection of platelet-rich plasma versus saline for treatment of olfactory dysfunction]. https://clinicaltrials.gov/show/NCT04406584 (first received 28 May 2020). [CENTRAL: CN-02124993]
NCT04414124 {published data only}
-
- NCT04414124. A clinical study to assess the natural history of COVID-19 and effects of KB109 and supportive self-care in outpatients with mild-to-moderate COVID-19 [A randomized, open label, prospective, parallel group study to assess the natural history of COVID-19 and effects of KB109 in addition to supportive self care (SSC) compared to SSC alone on measures of health in non-hospitalized patients with mild-moderate COVID-19]. https://clinicaltrials.gov/show/NCT04414124 (first received 4 June 2020).
NCT04427332 {published data only}
-
- NCT04427332. Smell and taste disorders in COVID-19 patients [Smell and taste disorders in COVID-19 patients: prospective observational study]. ClinicalTrials.gov (first received 2020).
NCT04458519 {published data only}
-
- NCT04458519. Efficacy of intranasal probiotic treatment to reduce severity of symptoms in COVID19 infection [Randomised single blinded clinical study of efficacy of intranasal probiotic treatment to reduce severity of symptoms in COVID19 infection]. https://clinicaltrials.gov/show/NCT04458519 (first received 7 July 2020).
NCT04474483 {published data only}
-
- NCT04474483. Safety and efficacy of melatonin in outpatients infected with COVID-19 [A pilot placebo-controlled randomized double-blind trial of melatonin in outpatients with COVID-19 infection]. https://clinicaltrials.gov/show/NCT04474483 (first received 16 July 2020).
NCT04513184 {published data only}
-
- NCT04513184. Randomized clinical trial of intranasal dexamethasone as an adjuvant in patients with COVID-19. https://clinicaltrials.gov/show/NCT04513184 (first received 14 August 2020). [CENTRAL: CN-02146132]
NCT04622891 {published data only}
-
- NCT04622891. Clarithromycin versus azithromycin in treatment of mild COVID-19 infection [Efficacy of clarithromycin in comparison to azithromycin in treatment of mild COVID-19 infection, randomized controlled trial]. https://clinicaltrials.gov/show/NCT04622891 (first received 10 November 2020). [CENTRAL: CN-02184257]
NCT04662086 {published data only}
-
- NCT04662086. COVID-19 Outpatient Pragmatic Platform Study (COPPS) - master protocol [COVID-19 Outpatient Pragmatic Platform Study (COPPS): a pragmatic multi-arm, adaptive, phase 2, blinded, randomized placebo-controlled platform trial to assess the efficacy of different investigational therapeutics in reducing time to disease resolution or viral load cessation, as compared to standard supportive care in outpatients with COVID-19]. https://clinicaltrials.gov/show/NCT04662086 (first received 10 December 2020).
Odorat‐Covid (NCT04598763) {published data only}
-
- NCT04598763. Evaluation of two methods of olfactory rehabilitation in post-viral loss of smell: classic and intensive. https://clinicaltrials.gov/show/NCT04598763 (first received 22 October 2020).
Patel 2021 {published data only}
-
- Patel ZM. Regarding use of topical steroids in patients with COVID-19-associated olfactory loss. JAMA Otolaryngology - Head and Neck Surgery 2021;147(1):110. - PubMed
Pinna 2020 {published data only}
Vaira 2020 {published data only}
-
- Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Cutrupi S, Salzano G, et al. Efficacy of corticosteroid therapy in the treatment of long- lasting olfactory disorders in COVID-19 patients. Rhinology 2020;59(1):21-5. [CENTRAL: CN-02210463] [EMBASE: 633663621] [PMID: ] - PubMed
Vroegop 2020 {published data only}
-
- Vroegop AV, Eeckels AS, Van Rompaey V, Vanden Abeele D, Schiappoli M, Alobid I, et al. COVID-19 and olfactory dysfunction - an ENT perspective to the current COVID-19 pandemic. B-ENT 2020;16(1):81-5.
References to ongoing studies
NCT04495816 {published data only}
-
- Lerner D, Garvey K, Arrighi-Allisan A, Filimonov A, Filip P, Liu K, et al. Letter to the editor: study summary - randomized control trial of omega-3 fatty acid supplementation for the treatment of COVID-19 related olfactory dysfunction. Trials 2020;21(1):942. [CENTRAL: CN-02200204] [EMBASE: 2007355105] [PMID: ] - PMC - PubMed
-
- NCT04495816. COVID-19 anosmia study. https://clinicaltrials.gov/show/NCT04495816 (first received 3 August 2020). [CENTRAL: CN-02145765]
NCT04528329 {published data only}
-
- NCT04528329. Anosmia and / or ageusia and early corticosteroid use [Time to recover of anosmia and / or ageusia and early corticosteroid use]. ClinicalTrials.gov (first received 2020).
NCT04530409 {published data only}
-
- NCT04530409. Timing of corticosteroids in COVID-19. https://clinicaltrials.gov/show/NCT04530409 (first received 28 August 2020).
NCT04569825 {published data only}
-
- NCT04569825. Effect of nasal steroid in the treatment of anosmia due to COVID-19 disease. https://clinicaltrials.gov/show/NCT04569825 (first received 30 September 2020). [CENTRAL: CN-02181592]
NCT04638673 {published data only}
-
- NCT04638673. NeuroCovid Rehab and Recovery Related to COVID-19 Diagnosis [Testing a wearable telemedicine-controllable taVNS device for NeuroCovid recovery and rehab]. https://clinicaltrials.gov/show/NCT04638673 (first received 20 November 2020).
NCT04657809 {published data only}
-
- NCT04657809. Clinical assessment of insulin fast dissolving film in treatment of post infection anosmia [Insulin fast dissolving film for intranasal delivery via olfactory region, a promising approach for the treatment of anosmia in COVID 19 patients: design, in-vitro characterization and clinical evaluation]. https://clinicaltrials.gov/show/NCT04657809 (first received 8 December 2020). - PubMed
Additional references
Abdelmaksoud 2021
-
- Abdelmaksoud AA, Ghweil AA, Hassan MH, Rashad A, Khodeary A, Aref ZF, et al. Olfactory disturbances as presenting manifestation among Egyptian patients with COVID-19: possible role of zinc. Biological Trace Element Research 2021 Jan 7 [Epub ahead of print]. [DOI: 10.1007/s12011-020-02546-5] - DOI - PMC - PubMed
Alexander 2006
Allen 2000
-
- Allen DB. Systemic effects of intranasal steroids: an endocrinologist’s perspective . Journal of Allergy and Clinical Immunology 2000;106(4):S179-90. - PubMed
Bigman 2020
Bilinska 2020
Boscolo‐Rizzo 2020
Boscolo‐Rizzo 2021
Butowt 2020
Cain 1988
-
- Cain WS, Gent JF, Goodspeed RB, Leonard G. Evaluation of olfactory dysfunction in the Connecticut Chemosensory Clinical Research Center. Laryngoscope 1988;98(1):83-8. - PubMed
Chary 2020
-
- Chary E, Carsuzaa F, Trijolet J-P, Capitaine A-L, Roncato-Saberan M, Fouet K, et al. Prevalence and recovery from olfactory and gustatory dysfunctions in COVID-19 infection: a prospective multicenter study. American Journal of Rhinology & Allergy 2020;34(5):686-93. [DOI: 10.1177/1945892420930954] - DOI - PMC - PubMed
Croy 2014
Dai 2016
Damm 2004
-
- Damm M, Temmel A, Welge-Lüssen A, Eckel HE, Kreft MP, Klussmann JP, et al. Olfactory dysfunctions. Epidemiology and therapy in Germany, Austria and Switzerland [Riechstörungen. Epidemiologie und Therapie in Deutschland, Osterreich und der Schweiz]. HNO 2004;52(2):112-20. - PubMed
Demoly 2008
Derin 2016
-
- Derin S, Koseoglu S, Sahin C, Sahan M. Effect of vitamin B12 deficiency on olfactory function. International Forum of Allergy & Rhinology 2016;6(10):1051-5. [PMID: ] - PubMed
Egger 1997
Erskine 2020
-
- Erskine SE, Philpott CM. An unmet need: patients with smell and taste disorders. Clinical Otolaryngology 2020;45(2):197-203. - PubMed
Fuccillo 2020
Gerkin 2020
Gudziol 2006
-
- Gudziol V, Lötsch J, Hähner A, Zahnert T, Hummel T. Clinical significance of results from olfactory testing. Laryngoscope 2006;116(10):1858-63. [PMID: ] - PubMed
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Handbook 2020
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Hopkins 2020a
-
- Hopkins C, Kumar N. Loss of sense of smell as a marker of COVID-19 infection. https://www.sforl.org/wp-content/uploads/2020/03/Loss-of-sense-of-smell-.... - PMC - PubMed
Hopkins 2020b
-
- Hopkins C, Burges Watson DL, Kelly C, Deary V, Smith BC. Managing long COVID: don't overlook olfactory dysfunction. BMJ 2020;370:m3736. - PubMed
Hopkins 2020c
Huart 2020
Hummel 2002
-
- Hummel T, Heilmann S, Hüttenbriuk KB. Lipoic acid in the treatment of smell dysfunction following viral infection of the upper respiratory tract. Laryngoscope 2002;112(11):2076-80. [PMID: ] - PubMed
Hummel 2016
-
- Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, et al. Position paper on olfactory dysfunction. Rhinology 2016;56(1):1-30. - PubMed
Hummel 2017
-
- Hummel T, Whitcroft KL, Rueter G, Haehner A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. European Archives of Oto-rhino-laryngology 2017;274(7):2819-25. [PMID: ] - PubMed
IRCT20210205050247N1
-
- IRCT20210205050247N1. The effect of olfactory training and vitamin A in the olfactory loss of patients with covid-19 [A comparative study of the effect of olfactory training and vitamin A in the olfactory loss of patients with covid-19]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20210205050247N1 (first received 2021).
IRCT20210311050671N1
-
- IRCT20210311050671N1. Evaluation of the effect of laser acupuncture on improving the sense of smell in patients with COVID-19 [Effect of auricular acupuncture with the laser in post-viral anosmia during the COVID-19 pandemic]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20210311050671N1 (first received 2021).
Kasiri 2021
Klopfenstein 2020
Landis 2003
-
- Landis BN, Hummel T, Hugentobler M, Giger R, Lacroix JS. Ratings of overall olfactory function. Chemical Senses 2003;28(8):691-4. - PubMed
Lechien 2020
-
- Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. European Archives of Oto-rhino-laryngology 2020;277(8):2251-61. [DOI: 10.1007/s00405-020-05965-1] - DOI - PMC - PubMed
Lefebvre 2020
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Lötsch 2019
Mao 2020
Marshall 2018
Martineau 2020
Mattos 2018
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; Sep 13-17, Cape Town, South Africa. 2017.
Meng 2020
Menni 2020
Mohamad 2021
-
- Mohamad SA, Badawi AM, Mansour HF. Insulin fast-dissolving film for intranasal delivery via olfactory region, a promising approach for the treatment of anosmia in COVID-19 patients: design, in-vitro characterization and clinical evaluation. International Journal of Pharmaceutics 2021;601:120600. - PubMed
NCT04764981
-
- NCT04764981. Olfactory training for olfactory dysfunction after coronavirus disease - 19 (COVID-19) [Clinical outcomes of olfactory training for treatment of olfactory dysfunction after COVID-19]. https://clinicaltrials.gov/show/NCT04764981 (first received 21 February 2021).
NCT04797936
-
- NCT04797936. BNO 1030 extract (Imupret) in the treatment of mild forms of COVID-19 [A randomized, open-label, multicentre, comparative study of therapeutic efficacy, safety, and tolerability of BNO 1030 extract, in the treatment of mild forms of COVID-19]. https://clinicaltrials.gov/show/NCT04797936 (first received 15 March 2021).
NCT04900415
-
- NCT04900415. Olfactory and neurosensory rehabilitation in COVID-19-related olfactory dysfunction [Olfactory and neurosensory rehabilitation in coronavirus 2019 (COVID-19)-related olfactory dysfunction (OD)]. https://clinicaltrials.gov/show/NCT04900415 (first received 25 May 2021).
NCT04951362
-
- NCT04951362. Role of Ivermectin nanosuspension as nasal spray in treatment of persistant post COVID19 anosmia [Studying the expected effect of Ivermectin nanosuspension as nasal spray upon post COVID19 persistant anosmia]. https://clinicaltrials.gov/show/NCT04951362 (first received 6 July 2021).
NCT04952389
-
- NCT04952389. Acupuncture therapy for COVID-related olfactory loss. https://clinicaltrials.gov/show/NCT04952389 (first received 7 July 2021).
NCT04959747
-
- NCT04959747. Acupuncture for olfactory dysfunction in infected COVID-19 patients [Acupuncture for olfactory dysfunction in infected COVID-19 patients: a randomized, sham-controlled clinical trial]. https://clinicaltrials.gov/show/NCT04959747 (first received 13 July 2021).
NCT04964414
-
- NCT04964414. Treatment of pediatric patients that lost sense of smell due to COVID-19 [Prospective randomized study of the treatment of pediatric patients that lost sense of smell due to COVID-19]. https://clinicaltrials.gov/show/NCT04964414 (first received 16 July 2021).
Noel‐Storr 2018
-
- Noel-Storr AH. The Project Transform Team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 June 18-20; Oxford, UK. 2018.
Parma 2020
Pekala 2016
Philpott 2014
Quint 2002
-
- Quint C, Temmel AF, Hummel T, Ehrenberger K. The quinoxaline derivative caroverine in the treatment of sensorineural smell disorders: a proof-of-concept study. Acta Oto-laryngologica 2002;122(8):877-81. [PMID: ] - PubMed
Rashid 2021
Reden 2011
-
- Reden J, Herting B, Lill K, Kern R, Hummel T. Treatment of postinfectious olfactory disorders with minocycline: a double-blind, placebo-controlled study. Laryngoscope 2011;121(3):679-82. [PMID: ] - PubMed
RevMan 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rocke 2020
Saussez 2020
Seiden 2001
-
- Seiden AM, Duncan HJ. The diagnosis of a conductive olfactory loss. Laryngoscope 2001;111(1):9-14. - PubMed
Sorokowska 2017
-
- Sorokowska A, Drechsler E, Karwowski M, Hummel T. Effects of olfactory training: a meta-analysis. Rhinology 2017;55(1):17-26. - PubMed
Spinato 2020
Thomas 2017
-
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al, Living Systematic Review Network. Living systematic reviews 2: combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. - PubMed
Tierney 2007
UMIN000043537
-
- UMIN000043537. Post COVID-19 anosmia [Post COVID-19 anosmia treatments - anosmia treatments]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000043537 (first received 2021).
Vent 2010
-
- Vent J, Wang DW, Damm M. Effects of traditional Chinese acupuncture in post-viral olfactory dysfunction. Otolaryngology - Head and Neck Surgery 2010;142(4):505-9. [PMID: ] - PubMed
von Bartheld 2020
Wallace 2017
Webster 2021a
Welge‐Luessen 2005
-
- Welge-Luessen A, Hummel T, Stojan T, Wolfensberger M. What is the correlation between ratings and measures of olfactory function in patients with olfactory loss? American Journal of Rhinology 2005;19(6):567-71. - PubMed
Yildiz 2021
-
- Yildiz E, Koca Yildiz S, Kuzu S, Günebakan Ç, Bucak A, Kahveci OK. Comparison of the healing effect of nasal saline irrigation with triamcinolone acetonide versus nasal saline irrigation alone in COVID-19 related olfactory dysfunction: a randomized controlled study. Indian Journal of Otolaryngology and Head and Neck Surgery 2021 Jul 10 [Epub ahead of print]. [DOI: 10.1007/s12070-021-02749-9] - DOI - PMC - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous