Rapid Highly-Efficient Digestion and Peptide Mapping of Adeno-Associated Viruses
- PMID: 34291903
- PMCID: PMC8743033
- DOI: 10.1021/acs.analchem.1c02117
Rapid Highly-Efficient Digestion and Peptide Mapping of Adeno-Associated Viruses
Erratum in
-
Correction to Rapid Highly-Efficient Digestion and Peptide Mapping of Adeno-Associated Viruses.Anal Chem. 2021 Dec 14;93(49):16734. doi: 10.1021/acs.analchem.1c04871. Epub 2021 Dec 1. Anal Chem. 2021. PMID: 34850630 Free PMC article. No abstract available.
Abstract
Adeno-associated viruses (AAVs) comprise an area of rapidly growing interest due to their ability to act as a gene delivery vehicle in novel gene therapy strategies and vaccine development. Peptide mapping is a common technique in the biopharmaceutical industry to confirm the correct sequence, product purity, post-translational modifications (PTMs), and stability. However, conventional peptide mapping is time-consuming and has proven difficult to reproduce with viral capsids because of their high structural stability and the suboptimal localization of trypsin cleavage sites in the AAV protein sequences. In this study, we present an optimized peptide mapping-based workflow that provides thorough characterization within 1 day. This workflow is also highly reproducible due to its simplicity having very few steps and is easy to perform proteolytic digestion utilizing thermally stable pepsin, which is active at 70 °C in acidic conditions. The acidic conditions of the peptic digestions drive viral capsid denaturation and improve cleavage site accessibility. We characterized the efficiency and ease of digestion through peptide mapping of the AAV2 viral capsid protein. Using nanoflow liquid chromatography coupled with tandem mass spectrometry, we achieved 100% sequence coverage of the low-abundance VP1 capsid protein with a digestion process taking only 10 min to prepare and 45 min to complete the digestion.
Conflict of interest statement
The authors declare no competing financial interest.
The mass spectrometry files have been deposited to the PRIDE Archive (
Figures
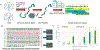


References
-
- Anguela XM; High KA Annu. Rev. Med 2019, 70, 273–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

