The immunodominant antibody response to Zika virus NS1 protein is characterized by cross-reactivity to self
- PMID: 34292314
- PMCID: PMC8302445
- DOI: 10.1084/jem.20210580
The immunodominant antibody response to Zika virus NS1 protein is characterized by cross-reactivity to self
Abstract
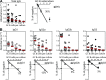
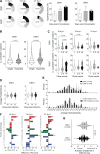
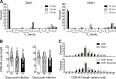
Besides antigen-specific responses to viral antigens, humoral immune response in virus infection can generate polyreactive and autoreactive antibodies. Dengue and Zika virus infections have been linked to antibody-mediated autoimmune disorders, including Guillain-Barré syndrome. A unique feature of flaviviruses is the secretion of nonstructural protein 1 (NS1) by infected cells. NS1 is highly immunogenic, and antibodies targeting NS1 can have both protective and pathogenic roles. In the present study, we investigated the humoral immune response to Zika virus NS1 and found NS1 to be an immunodominant viral antigen associated with the presence of autoreactive antibodies. Through single B cell cultures, we coupled binding assays and BCR sequencing, confirming the immunodominance of NS1. We demonstrate the presence of self-reactive clones in germinal centers after both infection and immunization, some of which present cross-reactivity with NS1. Sequence analysis of anti-NS1 B cell clones showed sequence features associated with pathogenic autoreactive antibodies. Our findings demonstrate NS1 immunodominance at the cellular level as well as a potential role for NS1 in ZIKV-associated autoimmune manifestations.
© 2021 Cavazzoni et al.
Conflict of interest statement
Disclosures: The authors declare no competing financial interests.
Figures









Comment in
-
A dark side to NS1 antibodies?J Exp Med. 2021 Sep 6;218(9):e20211348. doi: 10.1084/jem.20211348. Epub 2021 Jul 28. J Exp Med. 2021. PMID: 34319348 Free PMC article.
References
-
- Akey, D.L., Brown W.C., Dutta S., Konwerski J., Jose J., Jurkiw T.J., DelProposto J., Ogata C.M., Skiniotis G., Kuhn R.J., and Smith J.L.. 2014. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science. 343:881–885. 10.1126/science.1247749 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

