Pazopanib for severe bleeding and transfusion-dependent anemia in hereditary hemorrhagic telangiectasia
- PMID: 34292451
- PMCID: PMC8295629
- DOI: 10.1007/s10456-021-09807-4
Pazopanib for severe bleeding and transfusion-dependent anemia in hereditary hemorrhagic telangiectasia
Abstract
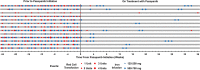
Hereditary hemorrhagic telangiectasia (HHT) is a rare angiogenic disorder causing chronic gastrointestinal bleeding, epistaxis, and severe anemia. Pazopanib is an oral multi-kinase angiogenesis inhibitor with promise to treat bleeding in HHT. We analyzed outcomes of HHT patients with the most severe bleeding causing RBC transfusion dependence treated on a predefined institutional pazopanib treatment pathway (with data collected retrospectively). The primary endpoint was achievement of transfusion independence. Secondary endpoints included hemoglobin, epistaxis severity score, RBC transfusion and iron infusion requirements, number of local hemostatic procedures, ferritin and transferrin saturation, compared using paired and repeated measures mean tests. Thirteen transfusion-dependent HHT patients received pazopanib [median (range) dose 150 (25-300) mg daily)] for a median of 22 months. All patients achieved transfusion independence. Compared with pretreatment, pazopanib increased mean hemoglobin by 4.8 (95% CI, 3.6-5.9) g/dL (7.8 vs. 12.7 g/dL, P < 0.0001) and decreased mean epistaxis severity score by 4.77 (3.11-6.44) points (7.20 vs. 2.43 points, P < 0.0001) after 12 months of treatment. Compared with 3 months of pretreatment, RBC transfusions decreased by 93% (median of 16.0 vs. 0.0 units, P < 0.0001) and elemental iron infusion decreased by 92% (median of 4500 vs. 0 mg, P = 0.005) during the first 3 months of treatment; improvements were maintained over time. Pazopanib was well-tolerated: hypertension, lymphocytopenia, and fatigue were the most common TEAEs. In conclusion, pazopanib was safe and effective to manage severe bleeding in HHT, liberating all patients from transfusion dependence and normalizing hematologic parameters at doses lower than used to treat malignancies. These findings require confirmation in a randomized trial.
Keywords: Anemia; Angiogenesis; Bleeding; Epistaxis; Gastrointestinal bleeding; HHT; Hereditary hemorrhagic telangiectasia; Iron deficiency; Osler-Weber-Rendu; Pazopanib.
© 2021. The Author(s), under exclusive licence to Springer Nature B.V.
Conflict of interest statement
Al-Samkari: consultancy (Agios, Dova, Argenx, Rigel, Sobi, Novartis), Research Funding to Institution (Agios, Dova, Amgen). All other authors have nothing to disclose.
Figures



References
-
- Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome) Am J Med Genet. 2000;91(1):66–67. doi: 10.1002/(SICI)1096-8628(20000306)91:1<66::AID-AJMG12>3.0.CO;2-P. - DOI - PubMed
-
- Letteboer TG, Mager HJ, Snijder RJ, Lindhout D, Ploos van Amstel HK, Zanen P, Westermann KJ. Genotype-phenotype relationship for localization and age distribution of telangiectases in hereditary hemorrhagic telangiectasia. Am J Med Genet A. 2008;146A(21):2733–2739. doi: 10.1002/ajmg.a.32243. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

