Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination
- PMID: 34292611
- PMCID: PMC8444807
- DOI: 10.1111/cup.14104
Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination
Abstract
Background: As more people become vaccinated against the SARS-CoV-2 virus, reports of delayed cutaneous hypersensitivity reactions are beginning to emerge.
Methods: In this IRB-approved retrospective case series, biopsy specimens of potential cutaneous adverse reactions from the Pfizer-BioNTech or Moderna mRNA vaccine were identified and reviewed. Clinical information was obtained through the requisition form, referring clinician, or medical chart review.
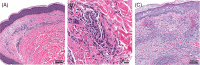
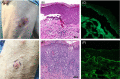
Results: Twelve cases were included. Histopathological features from two injection-site reactions showed a mixed-cell infiltrate with eosinophils and a spongiotic dermatitis with eosinophils. Three biopsy specimens came from generalized eruptions that showed interface changes consistent with an exanthematous drug reaction. Three biopsy specimens revealed a predominantly spongiotic pattern, consistent with eczematous dermatitis. Small-vessel vascular injury was seen in two specimens, which were diagnosed as urticarial vasculitis and leukocytoclastic vasculitis, respectively. There were two cases of new-onset bullous pemphigoid supported by histopathological examination and direct immunofluorescence studies. Eosinophils were seen in 10 cases.
Conclusions: Dermatopathologists should be aware of potential cutaneous adverse reactions to mRNA-based COVID-19 vaccines. Histopathological patterns include mixed-cell infiltrates, epidermal spongiosis, and interface changes. Eosinophils are a common finding but are not always present. Direct immunofluorescence studies may be helpful for immune-mediated cutaneous presentations such as vasculitis or bullous pemphigoid.
Keywords: COVID-19; cutaneous adverse reaction; vaccine.
© 2021 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

