Colon-Targeted Adhesive Hydrogel Microsphere for Regulation of Gut Immunity and Flora
- PMID: 34292669
- PMCID: PMC8456273
- DOI: 10.1002/advs.202101619
Colon-Targeted Adhesive Hydrogel Microsphere for Regulation of Gut Immunity and Flora
Abstract
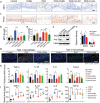
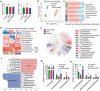
Intestinal immune homeostasis and microbiome structure play a critical role in the pathogenesis and progress of inflammatory bowel disease (IBD), whereas IBD treatment remains a challenge as the first-line drugs show limited therapeutic efficiency and great side effect. In this study, a colon-targeted adhesive core-shell hydrogel microsphere is designed and fabricated by the ingenious combination of advanced gas-shearing technology and ionic diffusion method, which can congregate on colon tissue regulating the gut immune-microbiota microenvironment in IBD treatment. The degradation experiment indicates the anti-acid and colon-targeted property of the alginate hydrogel shell, and the in vivo imaging shows the mucoadhesive ability of the thiolated-hyaluronic acid hydrogel core of the microsphere, which reduces the systematic exposure and prolongs the local drug dwell time. In addition, both in vitro and in vivo study demonstrate that the microsphere significantly reduces the secretion of pro-inflammatory cytokines, induces specific type 2 macrophage differentiation, and remarkably alleviates colitis in the mice model. Moreover, 16S ribosomal RNA sequencing reveals an optimized gut flora composition, probiotics including Bifidobacterium and Lactobacillus significantly augment, while the detrimental communities are inhibited, which benefits the intestinal homeostasis. This finding provides an ideal clinical candidate for IBD treatment.
Keywords: colitis; colon-targeted drug delivery; gut microbiota; hydrogel microsphere; oral administration.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Doherty G., Katsanos K. H., Burisch J., Allez M., Papamichael K., Stallmach A., Mao R., Berset I. P., Gisbert J. P., Sebastian S., Kierkus J., Lopetuso L., Szymanska E., Louis E., J. Crohns Colitis 2018, 12, 17. - PubMed
-
- Ng S. C., Shi H. Y., Hamidi N., Underwood F. E., Tang W., Benchimol E. I., Panaccione R., Ghosh S., Wu J. C. Y., Chan F. K. L., Sung J. J. Y., Kaplan G. G., Lancet 2018, 390, 2769. - PubMed
-
- Zhang Q., Tao H., Lin Y., Hu Y., An H., Zhang D., Feng S., Hu H., Wang R., Li X., Zhang J., Biomaterials 2016, 105, 206. - PubMed
-
- Hirten R. P., Iacucci M., Shah S., Ghosh S., Colombel J. F., Clin. Gastroenterol. Hepatol. 2018, 16, 1374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2020YFA0908200/National Key Research and Development Program of China
- 81930051/National Natural Science Foundation of China
- 81970489/National Natural Science Foundation of China
- 2020M681319/Postdoctoral Research Foundation of China
- 20171906/Shanghai Municipal Education commission-Gaofeng Clinical Medicine
LinkOut - more resources
Full Text Sources
