Optimizing testing for COVID-19 in India
- PMID: 34292931
- PMCID: PMC8297905
- DOI: 10.1371/journal.pcbi.1009126
Optimizing testing for COVID-19 in India
Abstract
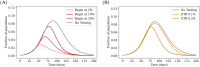
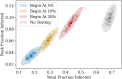
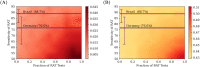
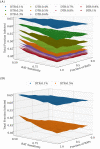
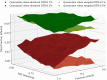
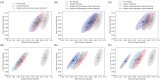
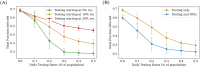
COVID-19 testing across India uses a mix of two types of tests. Rapid Antigen Tests (RATs) are relatively inexpensive point-of-care lateral-flow-assay tests, but they are also less sensitive. The reverse-transcriptase polymerase-chain-reaction (RT-PCR) test has close to 100% sensitivity and specificity in a laboratory setting, but delays in returning results, as well as increased costs relative to RATs, may vitiate this advantage. India-wide, about 49% of COVID-19 tests are RATs, but some Indian states, including the large states of Uttar Pradesh (pop. 227.9 million) and Bihar (pop. 121.3 million) use a much higher proportion of such tests. Here we show, using simulations based on epidemiological network models, that the judicious use of RATs can yield epidemiological outcomes comparable to those obtained through RT-PCR-based testing and isolation of positives, provided a few conditions are met. These are (a) that RAT test sensitivity is not too low, (b) that a reasonably large fraction of the population, of order 0.5% per day, can be tested, (c) that those testing positive are isolated for a sufficient duration, and that (d) testing is accompanied by other non-pharmaceutical interventions for increased effectiveness. We assess optimal testing regimes, taking into account test sensitivity and specificity, background seroprevalence and current test pricing. We find, surprisingly, that even 100% RAT test regimes should be acceptable, from both an epidemiological as well as a economic standpoint, provided the conditions outlined above are met.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Accuracy and cost description of rapid antigen test compared with reverse transcriptase-polymerase chain reaction for SARS-CoV-2 detection.Dan Med J. 2021 Jun 14;68(7):A03210217. Dan Med J. 2021. PMID: 34169830
-
Comparison of the SARS-CoV-2 Rapid antigen test to the real star Sars-CoV-2 RT PCR kit.J Virol Methods. 2021 Feb;288:114024. doi: 10.1016/j.jviromet.2020.114024. Epub 2020 Nov 20. J Virol Methods. 2021. PMID: 33227341 Free PMC article.
-
[Retrospective analysis of the performance of the SARS-CoV-2 rapid antigen detection test compared to the reference RT-PCR test].Ann Biol Clin (Paris). 2021 Apr 1;79(2):168-175. doi: 10.1684/abc.2021.1641. Ann Biol Clin (Paris). 2021. PMID: 33985935 French.
-
Diagnosis of SARS-CoV-2: A Review on the Current Scenario and Future Outlook.Acta Virol. 2020;64(4):396-408. doi: 10.4149/av_2020_402. Acta Virol. 2020. PMID: 32985200 Review.
-
Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis.J Med Virol. 2021 Jul;93(7):4523-4531. doi: 10.1002/jmv.26994. Epub 2021 May 3. J Med Virol. 2021. PMID: 33913533 Free PMC article.
Cited by
-
Exploring affordable and effective pandemic containment measures in lower-income countries with a spatial SEIR model: a case study in South Africa.BMC Public Health. 2025 Jul 23;25(1):2526. doi: 10.1186/s12889-025-23686-0. BMC Public Health. 2025. PMID: 40702473 Free PMC article.
-
Impact of antigen test target failure and testing strategies on the transmission of SARS-CoV-2 variants.Nat Commun. 2022 Oct 5;13(1):5870. doi: 10.1038/s41467-022-33460-0. Nat Commun. 2022. PMID: 36198689 Free PMC article.
-
Can Self-Administered Rapid Antigen Tests (RATs) Help Rural India? An Evaluation of the CoviSelf Kit as a Response to the 2019-2022 COVID-19 Pandemic.Diagnostics (Basel). 2022 Mar 6;12(3):644. doi: 10.3390/diagnostics12030644. Diagnostics (Basel). 2022. PMID: 35328197 Free PMC article.
-
Biotechnological Perspectives to Combat the COVID-19 Pandemic: Precise Diagnostics and Inevitable Vaccine Paradigms.Cells. 2022 Mar 31;11(7):1182. doi: 10.3390/cells11071182. Cells. 2022. PMID: 35406746 Free PMC article. Review.
-
The impact of earlier reopening to travel in the Western Pacific on SARS-CoV-2 transmission.IJID Reg. 2023 Mar;6:135-141. doi: 10.1016/j.ijregi.2022.11.013. Epub 2022 Nov 30. IJID Reg. 2023. PMID: 36466213 Free PMC article.
References
-
- Worldometers. India Coronavirus: 10,245,276 Cases and 148,470 Deaths–Worldometer; 2020. Available from: https://www.worldometers.info/coronavirus/country/india/.
-
- Menon GI. The Novel Coronavirus Variants and India’s Uncertain Future; 2021. Available from: https://science.thewire.in/health/sars-cov-2-variants-b117-b1617-india-s....
-
- Malani A, Shah D, Kang G, Lobo GN, Shastri J, Mohanan M, et al.. Seroprevalence of SARS-CoV-2 in slums and non-slums of Mumbai, India, during June 29-July 19, 2020. medRxiv. 2020; p. 2020.08.27.20182741.
-
- Delhi sero-survey results: Over 23% residents have coronavirus antibodies; 2020. Available from: https://www.livemint.com/news/india/delhi-sero-survey-results-over-23-re....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

