A review of the leishmanin skin test: A neglected test for a neglected disease
- PMID: 34292942
- PMCID: PMC8297750
- DOI: 10.1371/journal.pntd.0009531
A review of the leishmanin skin test: A neglected test for a neglected disease
Abstract
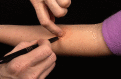
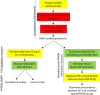
The leishmanin skin test (LST) has been used for decades to detect exposure and immunity to the parasite Leishmania, the causative agent of the neglected tropical disease leishmaniasis. In the LST, Leishmania antigen (leishmanin) is intradermally injected into the forearm. In an individual who has been previously infected, a delayed-type hypersensitivity (DTH) reaction results in a measurable induration at the site of the injection, indicating that previous exposure to Leishmania has resulted in the development of cell-mediated immunity. LST positivity is associated with long-lasting protective immunity against reinfection, most notably as reported for visceral leishmaniasis (VL). Despite efforts over the past few decades, leishmanin antigen is no longer produced under good manufacturing practice (GMP) conditions anywhere in the world. Consequently, the use of the LST in epidemiological studies has declined in favor of serological and molecular tests. In this review, we provide a historical overview of the LST and justification for the reintroduction of leishmanin. A GMP-grade leishmanin can be used to detect immunity in vivo by the LST and can be investigated for use in an interferon-γ release assay (IGRA), which may serve as an in vitro version of the LST. The LST will be a valuable tool for surveillance and epidemiological studies in support of the VL elimination programs and as a surrogate marker of immunity in vaccine clinical trials.
Methods: A review of the literature was conducted using PubMed as the primary database, with MeSH terms "leishmanin skin test" OR "Montenegro test" OR "Montenegro skin test." Articles written in English that describe the history or standardization of leishmanin, the use of leishmanin in an IGRA, or the use of the LST in epidemiological studies or vaccine trials were prioritized in our appraisal of the literature.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO. Leishmaniasis. Available from: https://www.who.int/health-topics/leishmaniasis.
-
- WHO. Neglected Tropical Diseases. Available from: https://www.who.int/neglected_diseases/diseases/en/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

